Efficient and Accurate 3D Thickness Measurement in Vessel Wall Imaging: Overcoming Limitations of 2D Approaches Using the Laplacian Method
- PMID: 39195157
- PMCID: PMC11354343
- DOI: 10.3390/jcdd11080249
Efficient and Accurate 3D Thickness Measurement in Vessel Wall Imaging: Overcoming Limitations of 2D Approaches Using the Laplacian Method
Abstract
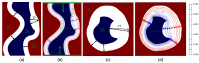
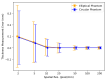
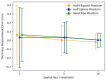
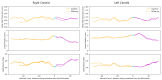
The clinical significance of measuring vessel wall thickness is widely acknowledged. Recent advancements have enabled high-resolution 3D scans of arteries and precise segmentation of their lumens and outer walls; however, most existing methods for assessing vessel wall thickness are 2D. Despite being valuable, reproducibility and accuracy of 2D techniques depend on the extracted 2D slices. Additionally, these methods fail to fully account for variations in wall thickness in all dimensions. Furthermore, most existing approaches are difficult to be extended into 3D and their measurements lack spatial localization and are primarily confined to lumen boundaries. We advocate for a shift in perspective towards recognizing vessel wall thickness measurement as inherently a 3D challenge and propose adapting the Laplacian method as an outstanding alternative. The Laplacian method is implemented using convolutions, ensuring its efficient and rapid execution on deep learning platforms. Experiments using digital phantoms and vessel wall imaging data are conducted to showcase the accuracy, reproducibility, and localization capabilities of the proposed approach. The proposed method produce consistent outcomes that remain independent of centerlines and 2D slices. Notably, this approach is applicable in both 2D and 3D scenarios. It allows for voxel-wise quantification of wall thickness, enabling precise identification of wall volumes exhibiting abnormal wall thickness. Our research highlights the urgency of transitioning to 3D methodologies for vessel wall thickness measurement. Such a transition not only acknowledges the intricate spatial variations of vessel walls, but also opens doors to more accurate, localized, and insightful diagnostic insights.
Keywords: Laplace’s equation; convolutions; vessel wall 3D thickness measurement; vessel wall imaging.
Conflict of interest statement
The authors declare no potential conflicts of interests.
Figures





References
-
- Stein J.H., Korcarz C.E., Hurst R.T., Lonn E., Kendall C.B., Mohler E.R., Najjar S.S., Rembold C.M., Post W.S. Use of Carotid Ultrasound to Identify Subclinical Vascular Disease and Evaluate Cardiovascular Disease Risk: A Consensus Statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force Endorsed by the Society for Vascular Medicine. J. Am. Soc. Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

