Cutting Edge: Low-dose Recombinant IL-2 Treatment Prevents Autoantibody Responses in Systemic Lupus Erythematosus via Regulatory T Cell-independent Depletion of T Follicular Helper Cells
- PMID: 39195194
- PMCID: PMC11606552
- DOI: 10.4049/jimmunol.2400264
Cutting Edge: Low-dose Recombinant IL-2 Treatment Prevents Autoantibody Responses in Systemic Lupus Erythematosus via Regulatory T Cell-independent Depletion of T Follicular Helper Cells
Abstract
The expansion of T follicular helper (Tfh) cells correlates with disease progression in human and murine systemic lupus erythematosus (SLE). Unfortunately, there are no therapies to deplete Tfh cells. Importantly, low-dose rIL-2-based immunotherapy shows potent immunosuppressive effects in SLE patients and lupus-prone mice, primarily attributed to the expansion of regulatory T cells (Tregs). However, IL-2 can also inhibit Tfh cell differentiation. In this study, we investigate the potential of low-dose rIL-2 to deplete Tfh cells and prevent autoantibody responses in SLE. Our data demonstrate that low-dose rIL-2 efficiently depletes autoreactive Tfh cells and prevents autoantibody responses in lupus-prone mice. Importantly, this immunosuppressive effect was independent of the presence of Tregs. The therapeutic potential of eliminating Tfh cells was confirmed by selectively deleting Tfh cells in lupus-prone mice. Our findings demonstrate the critical role of Tfh cells in promoting autoantibody responses and unveil, (to our knowledge), a novel Treg-independent immunosuppressive function of IL-2 in SLE.
Copyright © 2024 by The American Association of Immunologists, Inc.
Figures

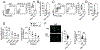
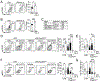
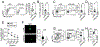
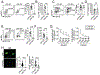
References
-
- Jenks SA, Cashman KS, Zumaquero E, Marigorta UM, Patel AV, Wang X, Tomar D, Woodruff MC, Simon Z, Bugrovsky R, Blalock EL, Scharer CD, Tipton CM, Wei C, Lim SS, Petri M, Niewold TB, Anolik JH, Gibson G, Lee FE, Boss JM, Lund FE, and Sanz I. 2018. Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity 49: 725–739 e726. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

