Importance of Autophagy Regulation in Glioblastoma with Temozolomide Resistance
- PMID: 39195222
- PMCID: PMC11353125
- DOI: 10.3390/cells13161332
Importance of Autophagy Regulation in Glioblastoma with Temozolomide Resistance
Abstract
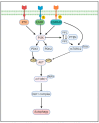
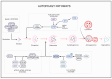
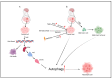
Glioblastoma (GBM) is the most aggressive and common malignant and CNS tumor, accounting for 47.7% of total cases. Glioblastoma has an incidence rate of 3.21 cases per 100,000 people. The regulation of autophagy, a conserved cellular process involved in the degradation and recycling of cellular components, has been found to play an important role in GBM pathogenesis and response to therapy. Autophagy plays a dual role in promoting tumor survival and apoptosis, and here we discuss the complex interplay between autophagy and GBM. We summarize the mechanisms underlying autophagy dysregulation in GBM, including PI3K/AKT/mTOR signaling, which is most active in brain tumors, and EGFR and mutant EGFRvIII. We also review potential therapeutic strategies that target autophagy for the treatment of GBM, such as autophagy inhibitors used in combination with the standard of care, TMZ. We discuss our current understanding of how autophagy is involved in TMZ resistance and its role in glioblastoma development and survival.
Keywords: autophagy; chemoresistance; glioblastoma; temozolomide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Rykkje A.M., Larsen V.A., Skjøth-Rasmussen J., Nielsen M.B., Carlsen J.F., Hansen A.E. Timing of Early Postoperative MRI following Primary Glioblastoma Surgery—A Retrospective Study of Contrast Enhancements in 311 Patients. Diagnostics. 2023;13:795. doi: 10.3390/diagnostics13040795. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

