Extracellular Vesicle Transplantation Is Beneficial for Acute Kidney Injury
- PMID: 39195224
- PMCID: PMC11352623
- DOI: 10.3390/cells13161335
Extracellular Vesicle Transplantation Is Beneficial for Acute Kidney Injury
Abstract
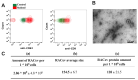
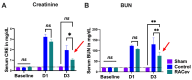
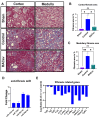
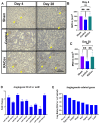
Under vasculogenic conditioning, certain pro-inflammatory subsets within peripheral blood mononuclear cells (PBMCs) undergo phenotypic transformation into pro-regenerative types, such as vasculogenic endothelial progenitor cells, M2 macrophages, and regulatory T cells. These transformed cells are collectively termed regeneration-associated cells (RACs). In this study, we aimed to investigate the therapeutic efficacy of RAC-derived extracellular vesicles (RACev) compared with a vehicle-treated group in the context of renal ischemia-reperfusion injury (R-IRI). Human PBMCs were cultured with defined growth factor cocktails for seven days to harvest RACs. EV quantity and size were characterized by nanoparticle tracking analysis. Notably, the systemic injection of RACev significantly decreased serum creatinine and blood urine nitrogen at day three compared to the control group. Histologically, the treatment group showed less fibrosis in the cortex and medullary areas (p < 0.04 and p < 0.01) compared to the control group. The CD31 staining confirmed enhanced capillary densities in the treatment group compared to the control group (p < 0.003). These beneficial effects were accompanied by angiogenesis, anti-fibrosis, anti-inflammation, and anti-apoptosis RACev miR delivery to ischemic injury to control inflammatory, endothelial mesenchymal transition, and hypoxia pathways. In vivo bioluminescence analysis demonstrated a preferential accumulation of RACev in the IR-injured kidney. The systemic transplantation of RACev beneficially restored kidney function by protecting from tissue fibrosis and through anti-inflammation, angiogenesis, and anti-apoptosis miR delivery to the ischemic tissue.
Keywords: angiogenesis; extracellular vesicles; miR; regeneration-associated cells; renal ischemia-reperfusion injury.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Hoste E.A.J., Bagshaw S.M., Bellomo R., Cely C.M., Colman R., Cruz D.N., Edipidis K., Forni L.G., Gomersall C.D., Govil D., et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensiv. Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. - DOI - PubMed
-
- Chawla L.S., Bellomo R., Bihorac A., Goldstein S.L., Siew E.D., Bagshaw S.M., Bittleman D., Cruz D., Endre Z., Fitzgerald R.L., et al. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 2017;13:241–257. doi: 10.1038/nrneph.2017.2. - DOI - PubMed
-
- The STARRT-AKI Investigators. For The Canadian Critical Care Trials Group. The Australian and New Zealand Intensive Care Society Clinical Trials Group. The United Kingdom Critical Care Research Group. The Canadian Nephrology Trials Network. The Irish Critical Care Trials Group. The STARRT-AKI Investigators for the Canadian Critical Care Trials Group. The United Kingdom Critical Care Research Group. The Canadian Nephrology Trials Network. The Irish Critical Care Trials Group Timing of Initiation of Renal-Replacement Therapy in Acute Kidney Injury. N. Engl. J. Med. 2020;383:240–251. doi: 10.1056/NEJMoa2000741. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

