Cancer-Associated-Fibroblast-Mediated Paracrine and Autocrine SDF-1/CXCR4 Signaling Promotes Stemness and Aggressiveness of Colorectal Cancers
- PMID: 39195225
- PMCID: PMC11352219
- DOI: 10.3390/cells13161334
Cancer-Associated-Fibroblast-Mediated Paracrine and Autocrine SDF-1/CXCR4 Signaling Promotes Stemness and Aggressiveness of Colorectal Cancers
Abstract
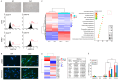
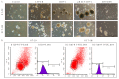
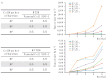
Colorectal cancer (CRC) is a leading cause of cancer mortality worldwide, and cancer-associated fibroblasts (CAFs) play a major role in the tumor microenvironment (TME), which facilitates the progression of CRC. It is critical to understand how CAFs promote the progression of CRC for the development of novel therapeutic approaches. The purpose of this study was to understand how CAF-derived stromal-derived factor-1 (SDF-1) and its interactions with the corresponding C-X-C motif chemokine receptor 4 (CXCR4) promote CRC progression. Our study focused on their roles in promoting tumor cell migration and invasion and their effects on the characteristics of cancer stem cells (CSCs), which ultimately impact patient outcomes. Here, using in vivo approaches and clinical histological samples, we analyzed the influence of secreted SDF-1 on CRC progression, especially in terms of tumor cell behavior and stemness. We demonstrated that CAF-secreted SDF-1 significantly enhanced CRC cell migration and invasion through paracrine signaling. In addition, the overexpression of SDF-1 in CRC cell lines HT29 and HCT-116 triggered these cells to generate autocrine SDF-1 signaling, which further enhanced their CSC characteristics, including those of migration, invasion, and spheroid formation. An immunohistochemical study showed a close relationship between SDF-1 and CXCR4 expression in CRC tissue, and this significantly affected patient outcomes. The administration of AMD3100, an inhibitor of CXCR4, reversed the entire phenomenon. Our results strongly suggest that targeting this signaling axis in CRC is a feasible approach to attenuating tumor progression, and it may, therefore, serve as an alternative treatment method to improve the prognosis of patients with CRC, especially those with advanced, recurrent, or metastatic CRC following standard therapy.
Keywords: C-X-C motif chemokine receptor 4 (CXCR4); cancer stem cells (CSCs); cancer-associated fibroblasts (CAFs); stromal-derived factor-1 (SDF-1); tumor microenvironment (TME).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

