Enhancing Maturation and Translatability of Human Pluripotent Stem Cell-Derived Cardiomyocytes through a Novel Medium Containing Acetyl-CoA Carboxylase 2 Inhibitor
- PMID: 39195229
- PMCID: PMC11352932
- DOI: 10.3390/cells13161339
Enhancing Maturation and Translatability of Human Pluripotent Stem Cell-Derived Cardiomyocytes through a Novel Medium Containing Acetyl-CoA Carboxylase 2 Inhibitor
Abstract
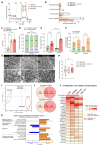
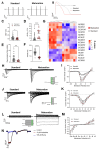
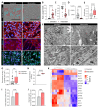
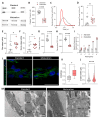
Human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs) constitute an appealing tool for drug discovery, disease modeling, and cardiotoxicity screening. However, their physiological immaturity, resembling CMs in the late fetal stage, limits their utility. Herein, we have developed a novel, scalable cell culture medium designed to enhance the maturation of hPSC-CMs. This medium facilitates a metabolic shift towards fatty acid utilization and augments mitochondrial function by targeting Acetyl-CoA carboxylase 2 (ACC2) with a specific small molecule inhibitor. Our findings demonstrate that this maturation protocol significantly advances the metabolic, structural, molecular and functional maturity of hPSC-CMs at various stages of differentiation. Furthermore, it enables the creation of cardiac microtissues with superior structural integrity and contractile properties. Notably, hPSC-CMs cultured in this optimized maturation medium display increased accuracy in modeling a hypertrophic cardiac phenotype following acute endothelin-1 induction and show a strong correlation between in vitro and in vivo target engagement in drug screening efforts. This approach holds promise for improving the utility and translatability of hPSC-CMs in cardiac disease modeling and drug discovery.
Keywords: acetyl-CoA carboxylase 2 (ACC2); cardiac hypertrophy; human pluripotent stem cell-derived cardiomyocyte (hPSC-CM) maturation; in vitro-to-in vivo correlation; translatable in vitro model.
Conflict of interest statement
C.C., J.C., S.T., S.E-H., M.M-G., S.N., G.M., M.R., M.S., I.S.B, Q-D.W., K.J. and D.S. are employees of AstraZeneca. B.N. is a previous employee of AstraZeneca and is now an employee of NovoNordisk. P.D. is an employee of SciCross AB. M.J., R.B.R., A.H., and J.S. declare no conflicts of interest.
Figures


References
-
- Seibertz F., Sutanto H., Dülk R., Pronto J.R.D., Springer R., Rapedius M., Liutkute A., Ritter M., Jung P., Stelzer L., et al. Electrophysiological and Calcium-Handling Development during Long-Term Culture of Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Basic. Res. Cardiol. 2023;118:14. doi: 10.1007/s00395-022-00973-0. - DOI - PMC - PubMed
-
- Lewandowski J., Rozwadowska N., Kolanowski T.J., Malcher A., Zimna A., Rugowska A., Fiedorowicz K., Łabędź W., Kubaszewski Ł., Chojnacka K., et al. The Impact of in Vitro Cell Culture Duration on the Maturation of Human Cardiomyocytes Derived from Induced Pluripotent Stem Cells of Myogenic Origin. Cell Transpl. 2018;27:1047–1067. doi: 10.1177/0963689718779346. - DOI - PMC - PubMed
-
- Wang L., Wada Y., Ballan N., Schmeckpeper J., Huang J., Rau C.D., Wang Y., Gepstein L., Knollmann B.C. Triiodothyronine and Dexamethasone Alter Potassium Channel Expression and Promote Electrophysiological Maturation of Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. J. Mol. Cell Cardiol. 2021;161:130–138. doi: 10.1016/j.yjmcc.2021.08.005. - DOI - PMC - PubMed
-
- Yang X., Rodriguez M., Pabon L., Fischer K.A., Reinecke H., Regnier M., Sniadecki N.J., Ruohola-Baker H., Murry C.E. Tri-Iodo-l-Thyronine Promotes the Maturation of Human Cardiomyocytes-Derived from Induced Pluripotent Stem Cells. J. Mol. Cell Cardiol. 2014;72:296–304. doi: 10.1016/j.yjmcc.2014.04.005. - DOI - PMC - PubMed
-
- Birket M.J., Ribeiro M.C., Kosmidis G., Ward D., Leitoguinho A.R., van de Pol V., Dambrot C., Devalla H.D., Davis R.P., Mastroberardino P.G., et al. Contractile Defect Caused by Mutation in MYBPC3 Revealed under Conditions Optimized for Human PSC-Cardiomyocyte Function. Cell Rep. 2015;13:733–745. doi: 10.1016/j.celrep.2015.09.025. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

