Direct Binding of Synaptopodin 2-Like Protein to Alpha-Actinin Contributes to Actin Bundle Formation in Cardiomyocytes
- PMID: 39195263
- PMCID: PMC11352367
- DOI: 10.3390/cells13161373
Direct Binding of Synaptopodin 2-Like Protein to Alpha-Actinin Contributes to Actin Bundle Formation in Cardiomyocytes
Abstract
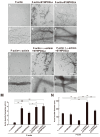
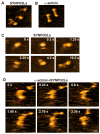
Synaptopodin 2-like protein (SYNPO2L) is localized in the sarcomere of cardiomyocytes and is involved in heart morphogenesis. However, the molecular function of SYNPO2L in the heart is not fully understood. We investigated the interaction of SYNPO2L with sarcomeric α-actinin and actin filaments in cultured mouse cardiomyocytes. Immunofluorescence studies showed that SYNPO2L colocalized with α-actinin and actin filaments at the Z-discs of the sarcomere. Recombinant SYNPO2La or SYNPO2Lb caused a bundling of the actin filaments in the absence of α-actinin and enhanced the α-actinin-dependent formation of actin bundles. In addition, high-speed atomic force microscopy revealed that SYNPO2La directly bound to α-actinin via its globular ends. The interaction between α-actinin and SYNPO2La fixed the movements of the two proteins on the actin filaments. These results strongly suggest that SYNPO2L cooperates with α-actinin during actin bundle formation to facilitate sarcomere formation and maintenance.
Keywords: SYNPO2L; actin; actinin; cardiomyocyte; sarcomere.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Van Eldik W., den Adel B., Monshouwer-Kloots J., Salvatori D., Maas S., van der Made I., Creemers E.E., Frank D., Frey N., Boontje N., et al. Z-disc protein CHAPb induces cardiomyopathy and contractile dysfunction in the postnatal heart. PLoS ONE. 2017;12:e0189139. doi: 10.1371/journal.pone.0189139. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

