New Insights into D-Aspartate Signaling in Testicular Activity
- PMID: 39195288
- PMCID: PMC11352307
- DOI: 10.3390/cells13161400
New Insights into D-Aspartate Signaling in Testicular Activity
Abstract
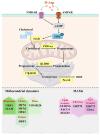
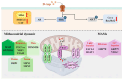
D-aspartate (D-Asp) is an amino acid found in high concentrations in the testis and pituitary gland. Increasing evidence suggests that D-Asp promotes spermatogenesis by activating testosterone production in the Leydig cells via LH release from the pituitary gland. In vitro studies indicate that D-Asp may also influence steroidogenesis and spermatogenesis through autocrine and paracrine signals. D-Asp enhances StAR and steroidogenic enzyme expressions, facilitating testicular cell proliferation via the GluR/ERK1/2 pathway. Moreover, it supports spermatogenesis by enhancing the mitochondrial function in spermatocytes, aiding in the metabolic shift during meiosis. Enhanced mitochondrial function, along with improved MAM stability and reduced ER stress, has been observed in Leydig and Sertoli cells treated with D-Asp, indicating potential benefits in steroidogenesis and spermatogenesis efficiency. Conversely, D-Asp exerts a notable anti-apoptotic effect in the testis via the AMPAR/AKT pathway, potentially mediated by antioxidant enzyme modulation to mitigate testicular oxidative stress. This review lays the groundwork for future investigations into the molecules promoting spermatogenesis by stimulating endogenous testosterone biosynthesis, with D-amino acids emerging as promising candidates.
Keywords: AKT; D-amino acids; D-aspartate; ERK1/2; MAMs; mitochondria; reproduction; spermatogenesis; steroidogenesis; testis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Karakawa S., Shimbo K., Yamada N., Mizukoshi T., Miyano H., Mita M., Lindner W., Hamase K. Simultaneous analysis of d-alanine, d-aspartic acid, and d-serine using chiral high-performance liquid chromatography-tandem mass spectrometry and its application to the rat plasma and tissues. J. Pharm. Biomed. Anal. 2015;115:123–129. doi: 10.1016/j.jpba.2015.05.024. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

