The Omission of Anthracycline Chemotherapy in Women with Early HER2-Negative Breast Cancer-A Systematic Review and Meta-Analysis
- PMID: 39195318
- PMCID: PMC11352883
- DOI: 10.3390/curroncol31080335
The Omission of Anthracycline Chemotherapy in Women with Early HER2-Negative Breast Cancer-A Systematic Review and Meta-Analysis
Abstract
Background: Anthracycline-taxane is the standard chemotherapy strategy for treating high-risk early breast cancer despite the potentially life-threatening adverse events caused by anthracyclines. Commonly, the combination of docetaxel and cyclophosphamide (TC) is considered an alternative option. However, the efficacy of TC compared to anthracycline-taxane chemotherapy is unclear. This study compares disease-free survival (DFS), overall survival (OS) and cardiotoxicity between adjuvant TC and anthracycline-taxane for stages I-III, HER2-negative breast cancer.
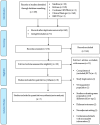
Methods: A systematic search on MEDLINE, Embase and Cochrane CENTRAL for randomized-controlled trials published until 11 March 2024, yielded 203 studies with 11,803 patients, and seven trials were included.
Results: TC results in little to no difference in DFS (HR 1.09, 95% CI 0.98-1.20; moderate-certainty of evidence); OS (1.02, 95% CI 0.89-1.16; high-certainty of evidence); and cardiotoxicity (RR 0.54, 95% CI 0.16-1.76; high-certainty of evidence), compared to anthracycline-taxane. In the subgroup analysis, patients with ≥4 lymph nodes had improved DFS from anthracycline-taxane over TC.
Conclusions: Overall, there was no difference between TC and anthracycline-taxane in DFS, OS and cardiotoxicity. In women with ≥4 nodes, anthracycline-taxane was associated with a substantial reduction in relapse events, compared to TC. Our study supports the current standard of practice, which is to use anthracycline-taxane and TC chemotherapy as a reasonable option in select cases.
Keywords: adjuvant; cyclophosphamide; docetaxel; doxorubicin; endocrine (hormone) receptor; epirubicin; human epidermal growth factor receptor 2 negative; invasive carcinoma; paclitaxel.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures











References
-
- Andre F., Ismaila N., Allison K.H., Barlow W.E., Collyar D.E., Damodaran S., Henry N.L., Jhaveri K., Kalinsky K., Kuderer N.M., et al. Biomarkers for adjuvant endocrine and chemotherapy in early-stage breast cancer: ASCO guideline update. J. Clin. Oncol. 2022;40:1816–1837. doi: 10.1200/JCO.22.00069. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

