Challenges and Opportunities in Developing an Oncology Clinical Trial Network in the United States Veterans Affairs Health Care System: The VA STARPORT Experience
- PMID: 39195341
- PMCID: PMC11353739
- DOI: 10.3390/curroncol31080358
Challenges and Opportunities in Developing an Oncology Clinical Trial Network in the United States Veterans Affairs Health Care System: The VA STARPORT Experience
Abstract
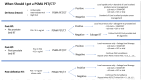
The United States Veterans Affairs (VA) Health Care System has a strong history of conducting impactful oncology randomized clinical trials (RCTs). We developed a phase II/III RCT to test the use of metastasis-directed therapy in Veterans with oligometastatic prostate cancer (OMPC)-the first VA RCT in OMPC that leverages novel imaging and advanced radiotherapy techniques. To accomplish this, we developed a clinical trial network to conduct the study. In this manuscript, we describe several challenges we encountered in study development/conduct and our strategies to address them, with the goal of helping investigators establish robust study networks to conduct clinical trials. In the study start-up, we encountered challenges in timely site activation, and leveraged project management to maximize efficiency. Additionally, there were several changes in the clinical paradigms in imaging and treatment that led to protocol amendments to ensure maximum equipoise, recruitment, and impact of the study. Specifically, we amended the trial to add de novo OMPC patients (from initially only recurrent OMPC) and expanded the study to allow up to 10 metastases (from initially five). Finally, in order to maintain local study team engagement, we developed initiatives to maximize collaboration and add value to the overall clinical program through study participation.
Keywords: oligometastasis; prostate cancer; radiotherapy; randomized clinical trial; recruitment; site selection; veterans.
Conflict of interest statement
The funders had no role in the design of this study, in the collection, analyses, or interpretation of the data, in the writing of this manuscript, or in the decision to publish the results. The following authors disclose their conflicts of interest: N.N. received research funding from Janssen, Lantheus, and Bayer, and personal fees from PrimeFour; M.R. received research funding from Merck, Lantheus, Janssen, Fusion, Novartis, and AstraZeneca, is a consultant for INmune Bio, Aveo, and Bayer, is a speaker for Bayer and Janssen, and is the inventor of patent W02018136792A1 “Inhibitors of the n-terminal domain of the androgen receptor”.
Figures


References
-
- Wolf G.T., Fisher S.G., Hong W.K., Hillman R., Spaulding M., Laramore G.E., Endicott J.W., McClatchey K., Henderson W.G. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N. Engl. J. Med. 1991;324:1685–1690. doi: 10.1056/nejm199106133242402. - DOI - PubMed
-
- Wilt T.J., Vo T.N., Langsetmo L., Dahm P., Wheeler T., Aronson W.J., Cooperberg M.R., Taylor B.C., Brawer M.K. Radical Prostatectomy or Observation for Clinically Localized Prostate Cancer: Extended Follow-up of the Prostate Cancer Intervention Versus Observation Trial (PIVOT) Eur. Urol. 2020;77:713–724. doi: 10.1016/j.eururo.2020.02.009. - DOI - PubMed
-
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Prostate Cancer Version 1.2024. [(accessed on 15 May 2024)]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
-
- VA Oncology Prostate Cancer Clinical Pathway V2.2024. [(accessed on 15 May 2024)]; Available online: https://www.cancer.va.gov/CANCER/assets/pdf/clinical-pathways/21/Prostat....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

