Voltage-Gated K+ Channel Modulation by Marine Toxins: Pharmacological Innovations and Therapeutic Opportunities
- PMID: 39195466
- PMCID: PMC11355921
- DOI: 10.3390/md22080350
Voltage-Gated K+ Channel Modulation by Marine Toxins: Pharmacological Innovations and Therapeutic Opportunities
Abstract
Bioactive compounds are abundant in animals originating from marine ecosystems. Ion channels, which include sodium, potassium, calcium, and chloride, together with their numerous variants and subtypes, are the primary molecular targets of the latter. Based on their cellular targets, these venom compounds show a range of potencies and selectivity and may have some therapeutic properties. Due to their potential as medications to treat a range of (human) diseases, including pain, autoimmune disorders, and neurological diseases, marine molecules have been the focus of several studies over the last ten years. The aim of this review is on the various facets of marine (or marine-derived) molecules, ranging from structural characterization and discovery to pharmacology, culminating in the development of some "novel" candidate chemotherapeutic drugs that target potassium channels.
Keywords: disease treating; marine toxins; peptides; voltage-gated potassium channels.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

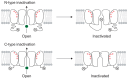

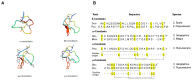


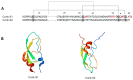
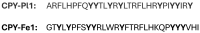
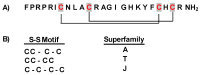
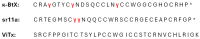


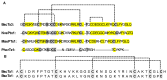




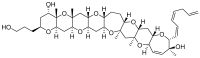
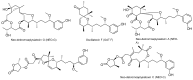
References
-
- Arias H. Marine Toxins Targeting Ion Channels. Mar. Drugs. 2006;4:37–69. doi: 10.3390/md403037. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

