ATP, the 31P Spectral Modulus, and Metabolism
- PMID: 39195552
- PMCID: PMC11356080
- DOI: 10.3390/metabo14080456
ATP, the 31P Spectral Modulus, and Metabolism
Abstract
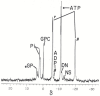
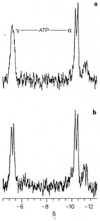
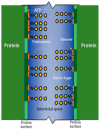
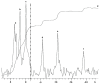
Adenosine triphosphate (ATP) has a high intracellular millimolar concentration (ca. 2.4 mM) throughout the phylogenetic spectrum of eukaryotes, archaea, and prokaryotes. In addition, the function of ATP as a hydrotrope in the prevention of protein aggregation and maintenance of protein solubilization is essential to cellular, tissue, and organ homeostasis. The 31P spectral modulus (PSM) is a measure of the health status of cell, tissue, and organ systems, as well as of ATP, and it is based on in vivo 31P nuclear magnetic resonance (31P NMR) spectra. The PSM is calculated by dividing the area of the 31P NMR integral curve representing the high-energy phosphates by that of the low-energy phosphates. Unlike the difficulties encountered in measuring organophosphates such as ATP or any other phosphorylated metabolites in a conventional 31P NMR spectrum or in processed tissue samples, in vivo PSM measurements are possible with NMR surface-coil technology. The PSM does not rely on the resolution of individual metabolite signals but uses the total area derived from each of the NMR integral curves of the above-described spectral regions. Calculation is based on a simple ratio of the high- and low-energy phosphate bands, which are conveniently arranged in the high- and low-field portions of the 31P NMR spectrum. In practice, there is essentially no signal overlap between these two regions, with the dividing point being ca. -3 δ. ATP is the principal contributor to the maintenance of an elevated PSM that is typically observed in healthy systems. The purpose of this study is to demonstrate that (1) in general, the higher the metabolic activity, the higher the 31P spectral modulus, and (2) the modulus calculation does not require highly resolved 31P spectral signals and thus can even be used with reduced signal-to-noise spectra such as those detected as a result of in vivo analyses or those that may be obtained during a clinical MRI examination. With increasing metabolic stress or maturation of metabolic disease in cells, tissues, or organ systems, the PSM index declines; alternatively, with decreasing stress or resolution of disease states, the PSM increases. The PSM can serve to monitor normal homeostasis as a diagnostic tool and may be used to monitor disease processes with and without interventional treatment.
Keywords: 31P nuclear magnetic resonance; 31P spectral modulus; ATP; hydrotrope; lens; protein aggregation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Glonek T. Applications of 31P NMR to biological system with emphasis on intact tissue determinations. In: Stec W.J., editor. Phosphorus Chemistry Directed towards Biology: Lectures Presented at the International Symposium Phosphorus Chemistry Directed towards Biology, Burzenin, Poland, 25–28 September 1979. Pergamon Press; Oxford, UK: 1980. pp. 157–174.
-
- Bárány M., Glonek T. Phosphorus-31 nuclear magnetic resonance of contractile systems. Methods Enzymol. 1982;85B:624–676. - PubMed
-
- Greiner J.V., Kopp S.J., Sanders D.R., Glonek T. Organophosphates of the crystalline lens: A nuclear magnetic resonance spectroscopic study. Investig. Ophthalmol. Vis. Sci. 1981;21:700–713. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

