Stroke and myocardial infarction induce neutrophil extracellular trap release disrupting lymphoid organ structure and immunoglobulin secretion
- PMID: 39195931
- PMCID: PMC11358010
- DOI: 10.1038/s44161-024-00462-8
Stroke and myocardial infarction induce neutrophil extracellular trap release disrupting lymphoid organ structure and immunoglobulin secretion
Abstract
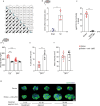
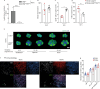
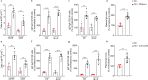
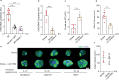
Post-injury dysfunction of humoral immunity accounts for infections and poor outcomes in cardiovascular diseases. Among immunoglobulins (Ig), IgA, the most abundant mucosal antibody, is produced by plasma B cells in intestinal Peyer's patches (PP) and lamina propria. Here we show that patients with stroke and myocardial ischemia (MI) had strongly reduced IgA blood levels. This was phenocopied in experimental mouse models where decreased plasma and fecal IgA were accompanied by rapid loss of IgA-producing plasma cells in PP and lamina propria. Reduced plasma IgG was detectable in patients and experimental mice 3-10 d after injury. Stroke/MI triggered the release of neutrophil extracellular traps (NETs). Depletion of neutrophils, NET degradation or blockade of NET release inhibited the loss of IgA+ cells and circulating IgA in experimental stroke and MI and in patients with stroke. Our results unveil how tissue-injury-triggered systemic NET release disrupts physiological Ig secretion and how this can be inhibited in patients.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
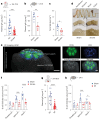
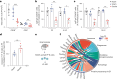

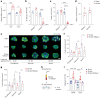
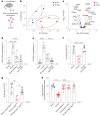
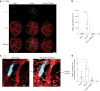

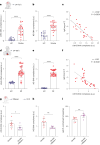

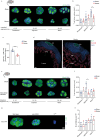

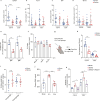

Comment in
-
Neutrophil extracellular traps trigger IgA loss after stroke and myocardial infarction.Nat Cardiovasc Res. 2024 May;3(5):496-497. doi: 10.1038/s44161-024-00465-5. Nat Cardiovasc Res. 2024. PMID: 39195933 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- SI 2650/1-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 3173/15-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- F31 DC007559/DC/NIDCD NIH HHS/United States
- 161L0272/Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (Federal Ministry for Education, Science, Research and Technology)
- 3173/12-1, 3173/13-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
