Cytotoxic T cells drive doxorubicin-induced cardiac fibrosis and systolic dysfunction
- PMID: 39196030
- PMCID: PMC12324073
- DOI: 10.1038/s44161-024-00507-y
Cytotoxic T cells drive doxorubicin-induced cardiac fibrosis and systolic dysfunction
Abstract
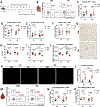
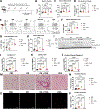
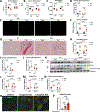
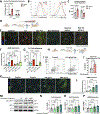
Doxorubicin, the most prescribed chemotherapeutic drug, causes dose-dependent cardiotoxicity and heart failure. However, our understanding of the immune response elicited by doxorubicin is limited. Here we show that an aberrant CD8+ T cell immune response following doxorubicin-induced cardiac injury drives adverse remodeling and cardiomyopathy. Doxorubicin treatment in non-tumor-bearing mice increased circulating and cardiac IFNγ+CD8+ T cells and activated effector CD8+ T cells in lymphoid tissues. Moreover, doxorubicin promoted cardiac CD8+ T cell infiltration and depletion of CD8+ T cells in doxorubicin-treated mice decreased cardiac fibrosis and improved systolic function. Doxorubicin treatment induced ICAM-1 expression by cardiac fibroblasts resulting in enhanced CD8+ T cell adhesion and transformation, contact-dependent CD8+ degranulation and release of granzyme B. Canine lymphoma patients and human patients with hematopoietic malignancies showed increased circulating CD8+ T cells after doxorubicin treatment. In human cancer patients, T cells expressed IFNγ and CXCR3, and plasma levels of the CXCR3 ligands CXCL9 and CXCL10 correlated with decreased systolic function.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors have no competing interests to declare.
Figures














References
-
- Henriksen PA Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart 104, 971–977 (2018). - PubMed
MeSH terms
Substances
Grants and funding
- K08 HL145019/HL/NHLBI NIH HHS/United States
- F30 HL162200/HL/NHLBI NIH HHS/United States
- HL162200/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL163172/HL/NHLBI NIH HHS/United States
- HL159907A/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- NIH R01 HL163172/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- Springboard Tier 1/Tufts University
- HL144477/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- F31 HL159907/HL/NHLBI NIH HHS/United States
- 906361/American Heart Association (American Heart Association, Inc.)
- U01 CA272268/CA/NCI NIH HHS/United States
- U01 CA224153/CA/NCI NIH HHS/United States
- 3R01HL144477-04S1/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL144477/HL/NHLBI NIH HHS/United States
- NIH K08 HL145019/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL165725/HL/NHLBI NIH HHS/United States
- 906561/American Heart Association (American Heart Association, Inc.)
- HL165725/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- NIH U01CA272268/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
