Cardiac troponin I directly binds and inhibits mitochondrial ATP synthase with a noncanonical role in the post-ischemic heart
- PMID: 39196031
- PMCID: PMC11700703
- DOI: 10.1038/s44161-024-00512-1
Cardiac troponin I directly binds and inhibits mitochondrial ATP synthase with a noncanonical role in the post-ischemic heart
Abstract
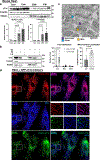
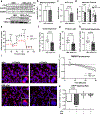
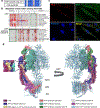
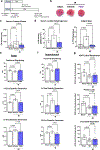
Cardiac troponin I (cTnI) is a key regulator of cardiomyocyte contraction. However, its role in mitochondria is unknown. Here we show that cTnI localized to mitochondria in the heart, inhibited mitochondrial functions when stably expressed in noncardiac cells and increased the opening of the mitochondrial permeability transition pore under oxidative stress. Direct, specific and saturable binding of cTnI to F1FO-ATP synthase was demonstrated in vitro using immune-captured ATP synthase and in cells using proximity ligation assay. cTnI binding doubled ATPase activity, whereas skeletal troponin I and several human pathogenic cTnI variants associated with familial hypertrophic cardiomyopathy did not. A rationally designed peptide, P888, inhibited cTnI binding to ATP synthase, inhibited cTnI-induced increase in ATPase activity in vitro and reduced cardiac injury following transient ischemia in vivo. We suggest that cTnI-bound ATP synthase results in lower ATP levels, and releasing this interaction during cardiac ischemia-reperfusion may increase the reservoir of functional mitochondria to reduce cardiac injury.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Figures




References
-
- Gibbs CL Cardiac energetics. Physiol. Rev 58, 174–254 (1978). - PubMed
-
- Suga H Ventricular energetics. Physiol. Rev 70, 247–277 (1990). - PubMed
-
- Opie LH Heart Physiology: From Cell to Circulation. (Lippincott Williams & Wilkins, 2004).
-
- Katrukha IA Human cardiac troponin complex. Structure and functions. Biochem. Biokhimiia 78, 1447–1465 (2013). - PubMed
MeSH terms
Substances
Grants and funding
- R01 HL052141/HL/NHLBI NIH HHS/United States
- HD099387/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- HL002066/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- 19POST34380299/American Heart Association (American Heart Association, Inc.)
- HL052141/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- HL09427411A1/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- Z01 HL002066/ImNIH/Intramural NIH HHS/United States
- 2021/09484-7/Fundação de Amparo à Pesquisa do Estado de São Paulo (São Paulo Research Foundation)
- K99 HD099387/HD/NICHD NIH HHS/United States
- HL006221/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
