Titin governs myocardial passive stiffness with major support from microtubules and actin and the extracellular matrix
- PMID: 39196092
- PMCID: PMC11358001
- DOI: 10.1038/s44161-023-00348-1
Titin governs myocardial passive stiffness with major support from microtubules and actin and the extracellular matrix
Abstract
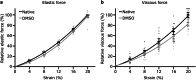
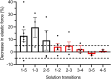
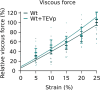
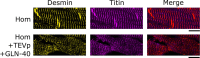
Myocardial passive stiffness is crucial for the heart's pump function and is determined by mechanical elements, including the extracellular matrix and cytoskeletal filaments; however, their individual contributions are controversially discussed and difficult to quantify. In this study, we targeted the cytoskeletal filaments in a mouse model, which enables the specific, acute and complete cleavage of the sarcomeric titin springs. We show in vitro that each cytoskeletal filament's stiffness contribution varies depending on whether the elastic or the viscous forces are considered and on strain level. Titin governs myocardial elastic forces, with the largest contribution provided at both low and high strain. Viscous force contributions are more uniformly distributed among the microtubules, titin and actin. The extracellular matrix contributes at high strain. The remaining forces after total target element disruption are likely derived from desmin filaments. Our findings answer longstanding questions about cardiac mechanical architecture and allow better targeting of passive myocardial stiffness in heart failure.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures










References
-
- van Heerebeek, L. et al. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation113, 966–1973 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
