Empagliflozin effects on iron metabolism as a possible mechanism for improved clinical outcomes in non-diabetic patients with systolic heart failure
- PMID: 39196095
- PMCID: PMC11358002
- DOI: 10.1038/s44161-023-00352-5
Empagliflozin effects on iron metabolism as a possible mechanism for improved clinical outcomes in non-diabetic patients with systolic heart failure
Abstract
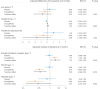
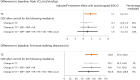
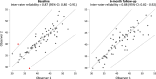
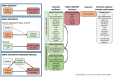
Sodium-glucose co-transporter-2 (SGLT2) inhibitors improve clinical outcomes in patients with heart failure (HF), but mechanisms of action are incompletely understood. In the EMPA-TROPISM trial, empagliflozin reversed cardiac remodeling and increased physical capacity in stable non-diabetic patients with systolic HF. Here we explore, post hoc, whether treatment effects in this cohort, comprising patients who had a high prevalence of iron deficiency, were related to iron metabolism. Myocardial iron content estimated by cardiac magnetic resonance T2* quantification increased after initiation of empagliflozin but not placebo (treatment effect: P = 0.01). T2* changes significantly correlated with changes in left ventricular volumes, mass and ejection fraction, peak oxygen consumption and 6-minute walking distance; concomitant changes in red blood cell indices were consistent with augmented hematopoiesis. Exploratory causal mediation analysis findings indicated that changes in myocardial iron content after treatment with empagliflozin may be an important mechanism to explain its beneficial clinical effects in patients with HF.ClinicalTrials.gov: NCT03485222 .
© 2023. The Author(s).
Conflict of interest statement
C.E.A. reports grant support, personal fees and/or non-financial support from Abbott, AstraZeneca, Boehringer Ingelheim and Eli Lilly, Medtronic, Novo Nordisk, Novartis, ResMed, Thermo Fisher Scientific and Vifor, all outside of the submitted work. C.G.S.-G. reports grant support from Merck, outside of the submitted work. C.M. reports personal fees from Amgen, Abbott, AstraZeneca, Berlin Chemie, Boehringer Ingelheim and Eli Lilly, Bristol-Myers Squibb, Edwards, Novartis, Novo Nordisk, Pharmacosmos and Servier, all outside of the submitted work. T.Z. reports previous funding from Vifor for research-related biobanking, outside of the submitted work. S.F. reports personal fees from Amgen Europe, AstraZeneca, Bayer Vital, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Merck Sharp & Dohme, Novartis, Pfizer, Sanofi, Servier and Vifor, all outside of the submitted work. G.E. reports personal fees and/or grant support from Abbott, Bayer, Boehringer Ingelheim and Eli Lilly, Novartis, ResMed and Vifor, all outside of the submitted work. J.J.B. reports grant support by Boehringer Ingelheim and Eli Lilly. All other authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
