A bispecific antibody approach for the potential prophylactic treatment of inherited bleeding disorders
- PMID: 39196196
- PMCID: PMC11358003
- DOI: 10.1038/s44161-023-00418-4
A bispecific antibody approach for the potential prophylactic treatment of inherited bleeding disorders
Abstract
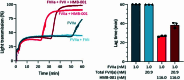
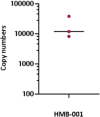
Inherited bleeding disorders such as Glanzmann thrombasthenia (GT) lack prophylactic treatment options. As a result, serious bleeding episodes are treated acutely with blood product transfusions or frequent, repeated intravenous administration of recombinant activated coagulation factor VII (rFVIIa). Here we describe HMB-001, a bispecific antibody designed to bind and accumulate endogenous FVIIa and deliver it to sites of vascular injury by targeting it to the TREM (triggering receptor expressed on myeloid cells)-like transcript-1 (TLT-1) receptor that is selectively expressed on activated platelets. In healthy nonhuman primates, HMB-001 prolonged the half-life of endogenous FVIIa, resulting in its accumulation. Mouse bleeding studies confirmed antibody-mediated potentiation of FVIIa hemostatic activity by TLT-1 targeting. In ex vivo models of GT, HMB-001 localized FVIIa on activated platelets and potentiated fibrin-dependent platelet aggregation. Taken together, these results indicate that HMB-001 has the potential to offer subcutaneous prophylactic treatment to prevent bleeds in people with GT and other inherited bleeding disorders, with a low-frequency dosing regimen.
© 2024. The Author(s).
Conflict of interest statement
P.S.G.: employee and shareholder of Hemab Therapeutics. M.Z.: no conflicts of interest. H.Ø.: employee and shareholder of Hemab Therapeutics. A.C.B.: employee and shareholder of Hemab Therapeutics. T.E.: employee and shareholder of Novo Nordisk. M.N.L.: employee and shareholder of Novo Nordisk. G.S.: employee and shareholder of Novo Nordisk. E.J.: employee and shareholder of Novo Nordisk. O.H.O.: received consultation fees from Hemab Therapeutics. E.H.N.O.: no conflicts of interest. I.-A.d.B.: employee of Sanquin Diagnostic Services. K.B.: employee of Sanquin Diagnostic Services. O.A.: received consultation fees from Hemab Therapeutics. C.J.R.: employee and shareholder of Hemab Therapeutics. S.E.B.: shareholder of Hemab Therapeutics. R.E.S.: the institution of R.E.S. has received speaker’s fees and/or research grants from Bayer, CSL Behring, Novartis, Novo Nordisk, Octapharma, Roche, Sobi and Takeda. B.S.: employee and shareholder of Hemab Therapeutics. R.T.U.: no conflicts of interest. J.H.F.: employee and shareholder of Hemab Therapeutics.
Figures








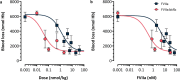


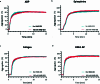
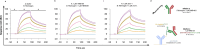

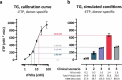
Comment in
-
Stopping the bleed when platelets don't stick.Nat Cardiovasc Res. 2024 Feb;3(2):100-101. doi: 10.1038/s44161-024-00421-3. Nat Cardiovasc Res. 2024. PMID: 39196195 No abstract available.
References
-
- Chitlur, M. B. et al. An update on rFVIIa use in females with rare bleeding disorders. Blood134, 1119 (2019). 10.1182/blood-2019-130298 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
