Evidence of Potential Natural Products for the Management of Hypertrophic Scars
- PMID: 39196306
- PMCID: PMC11359448
- DOI: 10.1177/2515690X241271948
Evidence of Potential Natural Products for the Management of Hypertrophic Scars
Abstract
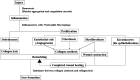
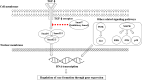
Hypertrophic scarring is an aberrant wound-healing response to reestablish dermal integrity after an injury and can cause significant abnormalities in physical, aesthetic, functional, and psychological symptoms, impacting the patient's quality of life. There is currently no gold standard for preventing and treating hypertrophic scars. Therefore, many researchers have attempted to search for antihypertrophic scar agents with greater efficacy and fewer side effects. Natural therapeutics are becoming attractive as potential alternative anti-scarring agents because of their high efficacy, safety, biocompatibility, low cost, and easy accessibility. This review demonstrates various kinds of natural product-based therapeutics, including onion, vitamin E, Gotu kola, green tea, resveratrol, emodin, curcumin, and others, in terms of their mechanisms of action, evidence of efficacy and safety, advantages, and disadvantages when used as anti-scarring agents. We reviewed the literature based on data from in vitro, in vivo, and clinical trials. A total of 23 clinical trials were identified in this review; most clinical trials were ranked as having uncertain results (level of evidence 2b; n = 16). Although these natural products showed beneficial effects in both in vitro and in vivo studies of potential anti-scarring agents, there was limited clinical evidence to support their efficacy due to the limited quality of the studies, with individual flaws including small sample sizes, poor randomization, and blinding, and short follow-up durations. More robust and well-designed clinical trials with large-scale and prolonged follow-up durations are required to clarify the benefits and risks of these agents.
Keywords: evidence-based herbal medicine; hypertrophic scar; mechanisms of action; natural products; scar.
Conflict of interest statement
Declaration of Conflicting InterestsThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
Topical treatments for hypertrophic scars.J Am Acad Dermatol. 2006 Dec;55(6):1024-31. doi: 10.1016/j.jaad.2006.03.022. Epub 2006 Sep 18. J Am Acad Dermatol. 2006. PMID: 17097399 Review.
-
Effect of Mederma on hypertrophic scarring in the rabbit ear model.Plast Reconstr Surg. 2002 Jul;110(1):177-83; discussion 184-6. doi: 10.1097/00006534-200207000-00029. Plast Reconstr Surg. 2002. PMID: 12087249
-
A comprehensive evidence-based review on the role of topicals and dressings in the management of skin scarring.Arch Dermatol Res. 2015 Aug;307(6):461-77. doi: 10.1007/s00403-015-1572-0. Epub 2015 Jun 5. Arch Dermatol Res. 2015. PMID: 26044054 Free PMC article. Review.
-
The efficacy of onion extract in the management of subsequent abdominal hypertrophic scar formation.J Wound Care. 2020 Oct 2;29(10):612-616. doi: 10.12968/jowc.2020.29.10.612. J Wound Care. 2020. PMID: 33052789 Clinical Trial.
-
Role of silicone derivative plus onion extract gel in presternal hypertrophic scar protection: a prospective randomized, double blinded, controlled trial.Int Wound J. 2012 Aug;9(4):397-402. doi: 10.1111/j.1742-481X.2011.00898.x. Epub 2011 Dec 14. Int Wound J. 2012. PMID: 22168750 Free PMC article. Clinical Trial.
Cited by
-
Comparative effectiveness of plant-derived compounds in keloid management: a review.Front Pharmacol. 2025 Jul 16;16:1576851. doi: 10.3389/fphar.2025.1576851. eCollection 2025. Front Pharmacol. 2025. PMID: 40741005 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

