Senescent cells inhibit mouse myoblast differentiation via the SASP-lipid 15d-PGJ2 mediated modification and control of HRas
- PMID: 39196610
- PMCID: PMC11357351
- DOI: 10.7554/eLife.95229
Senescent cells inhibit mouse myoblast differentiation via the SASP-lipid 15d-PGJ2 mediated modification and control of HRas
Abstract
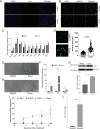
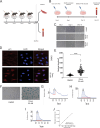
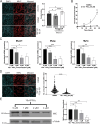
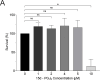
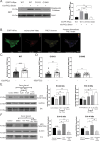
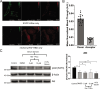
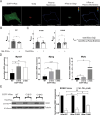
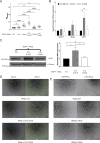
Senescent cells are characterized by multiple features such as increased expression of senescence-associated β-galactosidase activity (SA β-gal) and cell cycle inhibitors such as p21 or p16. They accumulate with tissue damage and dysregulate tissue homeostasis. In the context of skeletal muscle, it is known that agents used for chemotherapy such as Doxorubicin (Doxo) cause buildup of senescent cells, leading to the inhibition of tissue regeneration. Senescent cells influence the neighboring cells via numerous secreted factors which form the senescence-associated secreted phenotype (SASP). Lipids are emerging as a key component of SASP that can control tissue homeostasis. Arachidonic acid-derived lipids have been shown to accumulate within senescent cells, specifically 15d-PGJ2, which is an electrophilic lipid produced by the non-enzymatic dehydration of the prostaglandin PGD2. This study shows that 15d-PGJ2 is also released by Doxo-induced senescent cells as an SASP factor. Treatment of skeletal muscle myoblasts with the conditioned medium from these senescent cells inhibits myoblast fusion during differentiation. Inhibition of L-PTGDS, the enzyme that synthesizes PGD2, diminishes the release of 15d-PGJ2 by senescent cells and restores muscle differentiation. We further show that this lipid post-translationally modifies Cys184 of HRas in C2C12 mouse skeletal myoblasts, causing a reduction in the localization of HRas to the Golgi, increased HRas binding to Ras Binding Domain (RBD) of RAF Kinase (RAF-RBD), and activation of cellular Mitogen Activated Protein (MAP) kinase-Extracellular Signal Regulated Kinase (Erk) signaling (but not the Akt signaling). Mutating C184 of HRas prevents the ability of 15d-PGJ2 to inhibit the differentiation of muscle cells and control the activity of HRas. This work shows that 15d-PGJ2 released from senescent cells could be targeted to restore muscle homeostasis after chemotherapy.
Keywords: HRas; SASP; biochemistry; chemical biology; mouse; muscle differentiation; oxylipins; senescence.
© 2024, Pundlik et al.
Conflict of interest statement
SP, AB, AV, SS, MJ, RM, HL, MH, AR No competing interests declared
Figures
Update of
- doi: 10.1101/2023.12.23.573200
- doi: 10.7554/eLife.95229.1
- doi: 10.7554/eLife.95229.2
References
-
- Bihani T, Mason DX, Jackson TJ, Chen SC, Boettner B, Lin AW. Differential oncogenic ras signaling and senescence in tumor cells. Cell Cycle. 2004;3:1201–1207. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

