The RhoGEF protein Plekhg5 self-associates via its PH domain to regulate apical cell constriction
- PMID: 39196644
- PMCID: PMC11481697
- DOI: 10.1091/mbc.E24-04-0179
The RhoGEF protein Plekhg5 self-associates via its PH domain to regulate apical cell constriction
Abstract
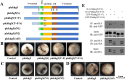
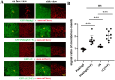
RhoGEFs are critical activators of Rho family small GTPases and regulate diverse biological processes, such as cell division and tissue morphogenesis. We reported previously that the RhoGEF gene plekhg5 controls apical constriction of bottle cells at the blastopore lip during Xenopus gastrulation, but the detailed mechanism of plekhg5 action is not understood in depth. In this study, we show that localization of Plekhg5 in the apical cortex depends on its N-terminal sequences and intact guanine nucleotide exchange activity, whereas the C-terminal sequences prevent ectopic localization of the protein to the basolateral compartment. We also reveal that Plekhg5 self-associates via its PH domain, and this interaction leads to functional rescue of two mutants that lack the N-terminal region and the guanine nucleotide exchange factor activity, respectively, in trans. A point mutation in the PH domain corresponding to a variant associated with human disease leads to loss of self-association and failure of the mutant to induce apical constriction. Taken together, our results suggest that PH-mediated self-association and N-terminal domain-mediated subcellular localization are both crucial for the function of Plekhg5 in inducing apical constriction.
Conflict of interest statement
Conflicts of interests: The authors declare no financial conflict of interest.
Figures




References
-
- Aigouy B, Farhadifar R, Staple DB, Sagner A, Roper JC, Julicher F, Eaton S (2010). Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell 142, 773–786. - PubMed
-
- Azzedine H, Zavadakova P, Plante-Bordeneuve V, Vaz Pato M, Pinto N, Bartesaghi L, Zenker J, Poirot O, Bernard-Marissal N, Arnaud Gouttenoire E, et al. (2013). PLEKHG5 deficiency leads to an intermediate form of autosomal-recessive Charcot-Marie-Tooth disease. Hum Mol Genet 22, 4224–4232. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

