Loss of the alpha subunit distal furin cleavage site blunts ENaC activation following Na+ restriction
- PMID: 39196791
- PMCID: PMC11384278
- DOI: 10.1113/JP286559
Loss of the alpha subunit distal furin cleavage site blunts ENaC activation following Na+ restriction
Abstract
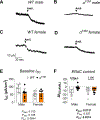
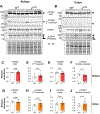
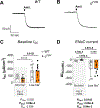
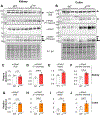
Epithelial Na+ channels (ENaCs) are activated by proteolysis of the α and γ subunits at specific sites flanking embedded inhibitory tracts. To examine the role of α subunit proteolysis in channel activation in vivo, we generated mice lacking the distal furin cleavage site in the α subunit (αF2M mice). On a normal Na+ control diet, no differences in ENaC protein abundance in kidney or distal colon were noted between wild-type (WT) and αF2M mice. Patch-clamp analyses revealed similar levels of ENaC activity in kidney tubules, while no physiologically relevant differences in blood chemistry or aldosterone levels were detected. Male αF2M mice did exhibit diminished ENaC activity in the distal colon, as measured by amiloride-sensitive short-circuit current (ISC). Following dietary Na+ restriction, WT and αF2M mice had similar natriuretic and colonic ISC responses to amiloride. However, single-channel activity was significantly lower in kidney tubules from Na+-restricted αF2M mice compared with WT littermates. ENaC α and γ subunit expression in kidney and distal colon were also enhanced in Na+-restricted αF2M vs. WT mice, in association with higher aldosterone levels. These data provide evidence that disrupting α subunit proteolysis impairs ENaC activity in vivo, requiring compensation in response to Na+ restriction. KEY POINTS: The epithelial Na+ channel (ENaC) is activated by proteolytic cleavage in vitro, but key questions regarding the role of ENaC proteolysis in terms of whole-animal physiology remain to be addressed. We studied the in vivo importance of this mechanism by generating a mouse model with a genetic disruption to a key cleavage site in the ENaC's α subunit (αF2M mice). We found that αF2M mice did not exhibit a physiologically relevant phenotype under normal dietary conditions, but have impaired ENaC activation (channel open probability) in the kidney during salt restriction. ENaC function at the organ level was preserved in salt-restricted αF2M mice, but this was associated with higher aldosterone levels and increased expression of ENaC subunits, suggesting compensation was required to maintain homeostasis. These results provide the first evidence that ENaC α subunit proteolysis is a key regulator of channel activity in vivo.
Keywords: Ussing chamber; aldosterone; amiloride; cell biology; ion channels; nephrology; patch clamp; proteolysis.
© 2024 The Author(s). The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures




References
-
- Al-Qusairi L, Basquin D, Roy A, Stifanelli M, Rajaram RD, Debonneville A, Nita I, Maillard M, Loffing J, Subramanya AR & Staub O. (2016). Renal tubular SGK1 deficiency causes impaired K+ excretion via loss of regulation of NEDD4–2/WNK1 and ENaC. Am J Physiol Renal Physiol 311, F330–342. - PMC - PubMed
-
- Amasheh S, Epple HJ, Mankertz J, Detjen K, Goltz M, Schulzke JD & Fromm M. (2000). Differential regulation of ENaC by aldosterone in rat early and late distal colon. Ann N Y Acad Sci 915, 92–94. - PubMed
-
- Asher C, Wald H, Rossier BC & Garty H. (1996). Aldosterone-induced increase in the abundance of Na+ channel subunits. Am J Physiol 271, C605–611. - PubMed
-
- Bhalla V & Hallows KR. (2008). Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol 19, 1845–1854. - PubMed
MeSH terms
Substances
Grants and funding
- F32DK136356/DK/NIDDK NIH HHS/United States
- U54 DK137329/DK/NIDDK NIH HHS/United States
- P30 DK079307/DK/NIDDK NIH HHS/United States
- R01 DK129285/DK/NIDDK NIH HHS/United States
- F32 DK136356/DK/NIDDK NIH HHS/United States
- R01 HL147818/HL/NHLBI NIH HHS/United States
- P30DK079307/DK/NIDDK NIH HHS/United States
- T32 DK061296/DK/NIDDK NIH HHS/United States
- T32DK061296/DK/NIDDK NIH HHS/United States
- R01DK125439/DK/NIDDK NIH HHS/United States
- U54DK137329/DK/NIDDK NIH HHS/United States
- R01HL147818/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01DK129285/DK/NIDDK NIH HHS/United States
- R01 DK125439/DK/NIDDK NIH HHS/United States
- R01 HL144941/HL/NHLBI NIH HHS/United States
- Gottschalk Research Scholar Grant/American Society of Nephrology (ASN)
LinkOut - more resources
Full Text Sources

