Development and Characterization of a Peptide-Bisphosphonate Nanoparticle for the Treatment of Breast Cancer
- PMID: 39196792
- PMCID: PMC11462496
- DOI: 10.1021/acs.molpharmaceut.4c00299
Development and Characterization of a Peptide-Bisphosphonate Nanoparticle for the Treatment of Breast Cancer
Abstract
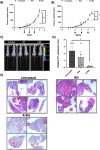
In women, breast cancer (BC) is the most common cancer, and despite advancements in diagnosis and treatment, 20-30% of early stage BC patients develop metastatic disease. Metastatic BC is deemed an incurable disease, which accounts for 90% of BC related deaths, with only 26% of metastatic patients reaching a 5 year survival rate. Therefore, there is an unmet need for the prevention or treatment of metastasis in early stage breast cancer patients. Bisphosphonates (BPs) are potent inhibitors of bone resorption and are extensively used for the prevention of osteoporosis and other skeletal disorders, as well as for the treatment of secondary bone cancer in BC patients. Furthermore, the direct anticancer activity of BPs has been established in primary tumor models. However, these studies were limited by the need for dosages far above the clinical range to overcome BPs' high affinity for bones and poor accumulation in the tumor itself, which leads to toxicity, including osteonecrosis of the jaw. To decrease BP dosage, increase bioavailability, and direct anticancer activity, we used the RALA (R-) peptide delivery system to form highly stable NPs with the nitrogen containing BP, risedronate (R-RIS). In vitro studies showed that, in comparison to RIS, R-RIS nanoparticles increased cytotoxicity and reduced metastatic features such as proliferation, migration, invasion, and adhesion of metastatic BC cells to bones. Furthermore, in an in vivo model, R-RIS had increased tumor accumulation while still maintaining similar bone accumulation to RIS alone. This increase in tumor accumulation corresponded with decreased tumor volume and lungs metastasis. R-RIS has great potential to be used in combination with standard of care chemotherapy for the treatment of primary BC and its metastasis while still having its bone resorption inhibiting properties.
Keywords: RALA; bisphosphates; breast cancer; nanomedicine; risedronate.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Early Breast Cancer (Preventing Recurrance and Improving Survival): Adjuvant Bisphosphonates [ES15]; National Institute for Health and Care Excellence, 2017.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

