Enhanced ECCD36 signaling promotes skeletal muscle insulin resistance in female mice
- PMID: 39196801
- PMCID: PMC11482271
- DOI: 10.1152/ajpendo.00246.2024
Enhanced ECCD36 signaling promotes skeletal muscle insulin resistance in female mice
Abstract
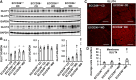
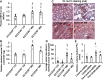
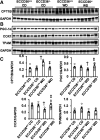
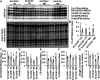
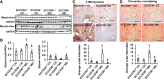
Consumption of a Western diet (WD) increases CD36 expression in vascular, hepatic, and skeletal muscle tissues promoting lipid metabolic disorders and insulin resistance. We further examined the role of endothelial cell-specific CD36 (ECCD36) signaling in contributing to skeletal muscle lipid metabolic disorders, insulin resistance, and their underlying molecular mechanisms. Female ECCD36 wild-type (ECCD36+/+) and knock-out (ECCD36-/-) mice, aged 6 wk, were provided with either a WD or a standard chow diet for a duration of 16 wk. ECCD36+/+ WD mice were characterized by elevated fasting plasma glucose and insulin levels, increased homeostatic model assessment for insulin resistance, and glucose intolerance that was blunted in ECCD36-/- mice. Improved insulin sensitivity in ECCD36-/- mice was characterized by increased phosphoinositide 3-kinases/protein kinase B signaling that further augmented glucose transporter type 4 expression and glucose uptake. Meanwhile, 16 wk of WD feeding also increased skeletal muscle free fatty acid (FFA) and lipid accumulation, without any observed changes in plasma FFA levels. These lipid metabolic disorders were blunted in ECCD36-/- mice. Moreover, ECCD36 also mediated in vitro palmitic acid-induced lipid accumulation in cultured ECs, subsequently leading to the release of FFAs into the culture media. Furthermore, consumption of a WD increased FFA oxidation, mitochondrial dysfunction, impaired mitochondrial respiratory, skeletal muscle fiber type transition, and fibrosis. These WD-induced abnormalities were blunted in ECCD36-/- mice. These findings demonstrate that endothelial-specific ECCD36 signaling participates in skeletal muscle FFA uptake, ectopic lipid accumulation, mitochondrial dysfunction, insulin resistance, and associated skeletal muscle dysfunction in diet-induced obesity.NEW & NOTEWORTHY ECCD36 exerts "extra endothelial cell" actions in skeletal muscle insulin resistance. ECCD36 is a major mediator of Western diet-induced lipid metabolic disorders and insulin resistance in skeletal muscle. Mitochondrial dysfunction is associated with diet-induced CD36 activation and related skeletal muscle insulin resistance.
Keywords: CD36; endothelial cells; insulin resistance; obesity; skeletal muscle.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Guglielmi V, Maresca L, D'Adamo M, Di Roma M, Lanzillo C, Federici M, Lauro D, Preziosi P, Bellia A, Sbraccia P. Age-related different relationships between ectopic adipose tissues and measures of central obesity in sedentary subjects. PLoS One 9: e103381, 2014. doi: 10.1371/journal.pone.0103381. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

