Determinants of pegivirus persistence, cross-species infection, and adaptation in the laboratory mouse
- PMID: 39196893
- PMCID: PMC11355568
- DOI: 10.1371/journal.ppat.1012436
Determinants of pegivirus persistence, cross-species infection, and adaptation in the laboratory mouse
Abstract
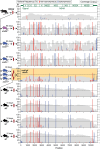
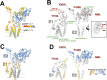
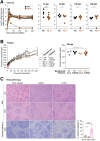
Viruses capable of causing persistent infection have developed sophisticated mechanisms for evading host immunity, and understanding these processes can reveal novel features of the host immune system. One such virus, human pegivirus (HPgV), infects ~15% of the global human population, but little is known about its biology beyond the fact that it does not cause overt disease. We passaged a pegivirus isolate of feral brown rats (RPgV) in immunodeficient laboratory mice to develop a mouse-adapted virus (maPgV) that established persistent high-titer infection in a majority of wild-type laboratory mice. maRPgV viremia was detected in the blood of mice for >300 days without apparent disease, closely recapitulating the hallmarks of HPgV infection in humans. We found a pro-viral role for type-I interferon in chronic infection; a lack of PD-1-mediated tolerance to PgV infection; and multiple mechanisms by which PgV immunity can be achieved by an immunocompetent host. These data indicate that the PgV immune evasion strategy has aspects that are both common and unique among persistent viral infections. The creation of maPgV represents the first PgV infection model in wild-type mice, thus opening the entire toolkit of the mouse host to enable further investigation of this persistent RNA virus infections.
Copyright: © 2024 Nennig et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
David O’Connor is a founder of Pathogenuity LLC. All other authors have no competing interests to disclose.
Figures






References
-
- Tacke M, Schmolke S, Schlueter V, Sauleda S, Esteban JI, Tanaka E, et al. Humoral immune response to the E2 protein of hepatitis G virus is associated with long-term recovery from infection and reveals a high frequency of hepatitis G virus exposure among healthy blood donors. Hepatology. 1997;26: 1626–1633. doi: 10.1002/hep.510260635 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

