Nanoparticle delivery of innate immune agonists combined with senescence-inducing agents promotes T cell control of pancreatic cancer
- PMID: 39196958
- PMCID: PMC11811823
- DOI: 10.1126/scitranslmed.adj9366
Nanoparticle delivery of innate immune agonists combined with senescence-inducing agents promotes T cell control of pancreatic cancer
Abstract
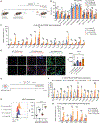
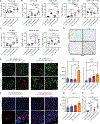
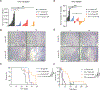
Pancreatic ductal adenocarcinoma (PDAC) has quickly risen to become the third leading cause of cancer-related death in the United States. This is in part because of its fibrotic tumor microenvironment (TME) that contributes to poor vascularization and immune infiltration and subsequent chemo- and immunotherapy failure. Here, we investigated an immunotherapy approach combining delivery of stimulator of interferon genes (STING) and Toll-like receptor 4 (TLR4) innate immune agonists by lipid-based nanoparticle (NP) coencapsulation with senescence-inducing RAS-targeted therapies, which can remodel the immune suppressive PDAC TME through the senescence-associated secretory phenotype. Treatment of transplanted and autochthonous PDAC mouse models with these regimens led to enhanced uptake of NPs by multiple cell types in the PDAC TME, induction of type I interferon and other proinflammatory signaling pathways, increased antigen presentation by tumor cells and antigen-presenting cells, and subsequent activation of both innate and adaptive immune responses. This two-pronged approach produced potent T cell-driven and type I interferon-mediated tumor regression and long-term survival in preclinical PDAC models dependent on both tumor and host STING activation. STING and TLR4-mediated type I interferon signaling was also associated with enhanced natural killer and CD8+ T cell immunity in human PDAC samples. Thus, combining localized immune agonist delivery with systemic tumor-targeted therapy can orchestrate a coordinated type I interferon-driven innate and adaptive immune response with durable antitumor efficacy against PDAC.
Figures




Update of
-
Nanoparticle delivery of innate immune agonists combines with senescence-inducing agents to mediate T cell control of pancreatic cancer.bioRxiv [Preprint]. 2023 Sep 18:2023.09.18.558307. doi: 10.1101/2023.09.18.558307. bioRxiv. 2023. Update in: Sci Transl Med. 2024 Aug 28;16(762):eadj9366. doi: 10.1126/scitranslmed.adj9366. PMID: 37790484 Free PMC article. Updated. Preprint.
References
-
- Siegel RL, Giaquinto AN, Jemal A, Cancer statistics, 2024. CA Cancer J Clin 74, 12–49 (2024). - PubMed
-
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM, Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366, 2455–2465 (2012). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

