Characterizing Long COVID in Children and Adolescents
- PMID: 39196964
- PMCID: PMC11339705
- DOI: 10.1001/jama.2024.12747
Characterizing Long COVID in Children and Adolescents
Abstract
Importance: Most research to understand postacute sequelae of SARS-CoV-2 infection (PASC), or long COVID, has focused on adults, with less known about this complex condition in children. Research is needed to characterize pediatric PASC to enable studies of underlying mechanisms that will guide future treatment.
Objective: To identify the most common prolonged symptoms experienced by children (aged 6 to 17 years) after SARS-CoV-2 infection, how these symptoms differ by age (school-age [6-11 years] vs adolescents [12-17 years]), how they cluster into distinct phenotypes, and what symptoms in combination could be used as an empirically derived index to assist researchers to study the likely presence of PASC.
Design, setting, and participants: Multicenter longitudinal observational cohort study with participants recruited from more than 60 US health care and community settings between March 2022 and December 2023, including school-age children and adolescents with and without SARS-CoV-2 infection history.
Exposure: SARS-CoV-2 infection.
Main outcomes and measures: PASC and 89 prolonged symptoms across 9 symptom domains.
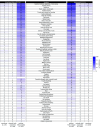
Results: A total of 898 school-age children (751 with previous SARS-CoV-2 infection [referred to as infected] and 147 without [referred to as uninfected]; mean age, 8.6 years; 49% female; 11% were Black or African American, 34% were Hispanic, Latino, or Spanish, and 60% were White) and 4469 adolescents (3109 infected and 1360 uninfected; mean age, 14.8 years; 48% female; 13% were Black or African American, 21% were Hispanic, Latino, or Spanish, and 73% were White) were included. Median time between first infection and symptom survey was 506 days for school-age children and 556 days for adolescents. In models adjusted for sex and race and ethnicity, 14 symptoms in both school-age children and adolescents were more common in those with SARS-CoV-2 infection history compared with those without infection history, with 4 additional symptoms in school-age children only and 3 in adolescents only. These symptoms affected almost every organ system. Combinations of symptoms most associated with infection history were identified to form a PASC research index for each age group; these indices correlated with poorer overall health and quality of life. The index emphasizes neurocognitive, pain, and gastrointestinal symptoms in school-age children but change or loss in smell or taste, pain, and fatigue/malaise-related symptoms in adolescents. Clustering analyses identified 4 PASC symptom phenotypes in school-age children and 3 in adolescents.
Conclusions and relevance: This study developed research indices for characterizing PASC in children and adolescents. Symptom patterns were similar but distinguishable between the 2 groups, highlighting the importance of characterizing PASC separately for these age ranges.
Conflict of interest statement
Figures




Comment in
References
-
- World Health Organization . Post COVID-19 condition (long COVID). December 7, 2022. Accessed February 27, 2024. https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-cond...
-
- Centers for Disease Control and Prevention (CDC) . Long COVID . Accessed February 27, 2024. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html

