Discovery of VU6007496: Challenges in the Development of an M1 Positive Allosteric Modulator Backup Candidate
- PMID: 39197083
- PMCID: PMC11413853
- DOI: 10.1021/acschemneuro.4c00508
Discovery of VU6007496: Challenges in the Development of an M1 Positive Allosteric Modulator Backup Candidate
Abstract
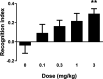
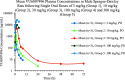
Herein we report progress toward a backup clinical candidate to the M1 positive allosteric modulator (PAM) VU319/ACP-319. Scaffold-hopping from the pyrrolo[2,3-b]pyridine-based M1 PAM VU6007477 to isomeric pyrrolo[3,2-b]pyridine and thieno[3,2-b]pyridine congeners identified several backup contenders. Ultimately, VU6007496, a pyrrolo[3,2-b]pyridine, advanced into late stage profiling, only to be plagued with unanticipated, species-specific metabolism and active/toxic metabolites which were identified in our phenotypic seizure liability in vivo screen, preventing further development. However, VU6007496 proved to be a highly selective and CNS penetrant M1 PAM, with minimal agonism, that displayed excellent multispecies IV/PO pharmacokinetics (PK), CNS penetration, no induction of long-term depression (or cholinergic toxicity) and robust efficacy in novel object recognition (minimum effective dose = 3 mg/kg p.o.). Thus, VU6007496 can serve as another valuable in vivo tool compound in rats and nonhuman primates, but not mouse, to study selective M1 activation.
Keywords: cognition; metabolism; muscarinic acetylcholine receptor subtype 1 (M1); positive allosteric modulator (PAM).
Conflict of interest statement
The authors declare the following competing financial interest(s): We hold U.S. patents on M1 PAMs (the chemical series in this article are no longer under development) and are working with Acadia Pharmaceuticals on new, distinct chemical matter.
Figures





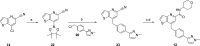







References
-
- Bodick N. C.; Offen W. W.; Levey A. I.; Cutler N. R.; Gauther S. G.; Satlin A.; Shannon H. E.; Tollefson G. D.; Rasmussen K.; Bymaster F. P.; Hurley D. J.; et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch. Neurol. 1997, 54 (4), 465–473. 10.1001/archneur.1997.00550160091022. - DOI - PubMed
-
- Shekhar A.; Potter W. Z.; Lightfoot J.; Lienemann D.; Dube S.; Mallinckrodt C.; Bymaster F. P.; McKinzie D. L.; Felder C. C. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry. 2008, 165, 1033–1039. 10.1176/appi.ajp.2008.06091591. - DOI - PubMed
-
- Melancon B. J.; Tarr J. C.; Panarese J. D.; Wood M. R.; Lindsley C. W. Allosteric modulation of the M1 muscarinic acetylcholine receptor: improving cognition and a potential treatment for schizophrenia and Alzheimer’s disease. Drug Discovery Today 2013, 18 (23–24), 1185–1199. 10.1016/j.drudis.2013.09.005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

