Effect of Ubrogepant on Patient-Reported Outcomes When Administered During the Migraine Prodrome: Results From the Randomized PRODROME Trial
- PMID: 39197113
- PMCID: PMC11379431
- DOI: 10.1212/WNL.0000000000209745
Effect of Ubrogepant on Patient-Reported Outcomes When Administered During the Migraine Prodrome: Results From the Randomized PRODROME Trial
Abstract
Background and objectives: Ubrogepant is a calcitonin gene-related peptide receptor antagonist approved for the acute treatment of migraine. The PRODROME trial previously demonstrated that ubrogepant treatment during prodrome prevents the onset of moderate or severe headache. In this analysis of the PRODROME trial, the benefits of ubrogepant treatment during the prodrome on patient-reported outcomes (PROs) are evaluated.
Methods: PRODROME was a multicenter, randomized, double-blind, placebo-controlled, crossover trial that enrolled adults who experienced 2-8 migraine attacks per month with moderate-severe headache pain. Eligible participants treated 2 qualifying prodrome events, defined as a migraine attack with prodromal symptoms when the participant was confident a headache would follow within 1-6 hours. Participants were randomized to treatment sequence A (placebo then ubrogepant 100 mg) or sequence B (ubrogepant 100 mg then placebo). This analysis evaluated the ability to function normally over 24 hours (secondary end point) and at specific time points after dose (additional end point). Other PRO end points included activity limitation over 24 hours and satisfaction with study medication at 8 and 24 hours.
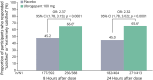
Results: Of 518 randomized participants, 477 comprised the modified intent-to-treat population. After treatment of qualifying prodrome events, a significantly greater ability to function normally over 24 hours was observed for participants after treatment with ubrogepant 100 mg compared with placebo (odds ratio [OR] 1.66, 95% CI 1.40-1.96; p < 0.0001). As early as 2 hours after dose, a greater proportion of ubrogepant-treated participants reported "no disability, able to function normally" compared with placebo (OR 1.76, 95% CI 1.32-2.35; nominal p = 0.0001). Ubrogepant administered during the prodrome was also associated with a greater reduction in activity limitations over 24 hours after dose (OR 2.07, 95% CI 1.61-2.67; nominal p < 0.0001). At 8 and 24 hours after dose, rates of being "satisfied" or "extremely satisfied" were greater for ubrogepant than for placebo (8 hours: OR 2.37, 95% CI 1.78-3.15; nominal p < 0.0001; 24 hours: OR 2.32, 95% CI 1.78-3.02; nominal p < 0.0001).
Discussion: Ubrogepant 100 mg administered during the prodrome was associated with significantly greater ability to function normally, greater reduction in activity limitations over 24 hours, and greater satisfaction with study medication, compared with placebo.
Trial registration information: ClinicalTrials.gov NCT04492020. Submitted: July 27, 2020; first patient enrolled: August 21, 2020. clinicaltrials.gov/ct2/show/NCT04492020.
Classification of evidence: This study provides Class II evidence that taking ubrogepant 100 mg during a migraine prodrome allows more patients to function normally over the next 24 hours.
Conflict of interest statement
R.B. Lipton has received research support from the National Institutes of Health, the FDA, and the National Headache Foundation, serves as consultant for, advisory board member of, or has received honoraria or research support from AbbVie/Allergan, Amgen, Biohaven, electroCore, Eli Lilly, GlaxoSmithKline, Lundbeck, Merck, Novartis, Teva, Vector, and Vedanta Research, receives royalties from Wolff's Headache, 8th edition (Oxford University Press, 2009), and Informa, and holds stock/options in Axon, Biohaven, Cooltech, and Manistee. A.M. Harriott has received personal compensation for serving as an officer or member of the Board of Directors for Headache Cooperative of New England, their institution has received research support from electroCore, and has a non-compensated relationship as an author with AbbVie that is relevant to AAN interests or activities. D.W. Dodick reports the following conflicts: Consulting: Amgen, Atria, CapiThera Ltd., Cerecin, Ceruvia Lifesciences LLC, CoolTech, Ctrl M, Allergan, AbbVie, Biohaven, GlaxoSmithKline, Lundbeck, Eli Lilly, Novartis, Impel, Satsuma, Theranica, WL Gore, Genentech, Nocira, Perfood, Praxis, AYYA Biosciences, Revance, and Pfizer. Honoraria: American Academy of Neurology, the Headache Cooperative of the Pacific, the Canadian Headache Society, MF Med Ed Research, Biopharm Communications, CEA Group Holding Company (Clinical Education Alliance LLC), Teva (speaking), Amgen (speaking), Eli Lilly (speaking), Lundbeck (speaking), Pfizer (speaking), Vector Psychometric Group, Clinical Care Solutions, CME Outfitters, Curry Rockefeller Group, DeepBench, Global Access Meetings, KLJ Associates, Academy for Continued Healthcare Learning, Majallin LLC, Medlogix Communications, Medica Communications LLC, MJH Lifesciences, Miller Medical Communications, WebMD Health/Medscape, Wolters Kluwer, Oxford University Press, and Cambridge University Press. Non-profit board membership: American Brain Foundation, American Migraine Foundation, ONE Neurology, Precon Health Foundation, International Headache Society Global Patient Advocacy Coalition, Atria Health Collaborative, Arizona Brain Injury Alliance, and the Domestic Violence HOPE Foundation/Panfila. Research support: Department of Defense, National Institutes of Health, the Henry Jackson Foundation, the Sperling Foundation, the American Migraine Foundation, and the Patient Centered Outcomes Research Institute (PCORI). Stock options/shareholder/patents/board of directors: Ctrl M (options), Aural Analytics (options), ExSano (options), Palion (options), Man and Science, Healint (options), Theranica (options), Second Opinion/Mobile Health (options), Epien (options/board), Nocira (options), Matterhorn (shares/board), Ontologics (shares/board), King-Devick Technologies (options/board), Precon Health (options/board), AYYA Biosciences (options), Axon Therapeutics (options/board), Cephalgia Group (options/board), Atria Health (options/employee). Patent 17189376.1-1466:vTitle: Onabotulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis (Nonroyalty bearing). Patent application submitted: Synaquell (Precon Health). J.Y. Ma, J.H. Smith, J. Stokes, P. Gandhi, K. Jariwala-Parikh, G.S. Jensen, and J.M. Trugman are employees of AbbVie and may hold AbbVie stock. Go to
Figures



References
-
- Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z; Lifting The Burden the Global Campaign against Headache. Migraine remains second among the world's causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020;21(1):137. doi:10.1186/s10194-020-01208-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
