Evidence-Based Clinical Guidelines for Chronic Diarrhea 2023
- PMID: 39197422
- PMCID: PMC11633876
- DOI: 10.1159/000541121
Evidence-Based Clinical Guidelines for Chronic Diarrhea 2023
Abstract
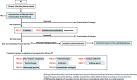
The Japan Gastroenterological Association (JGA) published the first version of clinical guidelines for chronic diarrhea 2023. These guidelines describe the definition, classification, diagnostic criteria, diagnostic testing methods, epidemiology, pathophysiology, and treatment of chronic diarrhea, and provide flowcharts for the diagnosis and treatment of chronic diarrhea based on the latest evidence. Treatment for chronic diarrhea begins by distinguishing secondary chronic constipation with a clear etiology, such as drug-induced diarrhea, food-induced diarrhea, systemic disease-associated diarrhea, infection-associated diarrhea, organic disease-associated diarrhea, and bile acid diarrhea. The first line of treatment for chronic diarrhea in the narrow sense, defined in these guidelines as functional diarrhea in routine medical care, is lifestyle modification and dietary therapy. The first medicines to be considered for oral treatment are probiotics for regulating the gut microbiome and anti-diarrheals. Other medications, such as 5HT3 receptor antagonists, anticholinergics, Kampo medicine, psychotherapy, antibiotics, bulking agents, adrenergic agonists, and somatostatin analogs, lack sufficient evidence for their use, highlighting a challenge for future research. This Clinical Guidelines for Chronic Diarrhea 2023, which provides the best clinical strategies for treating chronic diarrhea in Japan, will also be useful for medical treatment worldwide.
Keywords: Chronic diarrhea; Diarrhea-predominant irritable bowel syndrome; Functional diarrhea; Guidelines.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The following were the competing interests of guideline development committee members, guideline committee members, and guideline Evaluation Committee members. Any financial relationship with enterprises, businesses or academic institutions in the subject matter or materials discussed in the manuscript are as follows: (1) those from which the authors, the spouse, partner or immediate relatives of the authors have received individually any income, honoraria or any other type of remuneration; Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company, EA Pharma Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., AstraZeneca K. K., Olympus Corporation, JIMRO Co., Ltd., AbbVie GK, KYORIN Pharmaceutical Co., Ltd., Viatris Inc., Astellas Pharma Inc., Gilead Sciences, Inc., Mitsubishi Tanabe Pharma Corporation, Pfizer Japan Inc., Mochida Pharmaceutical Co., Ltd., Janssen Pharmaceutical K. K., Tsumura and Co., Towa Pharmaceutical Co., Ltd., Bristol-Myers Squibb K. K., Kowa Pharmaceutical Co., Ltd., Taisho Pharmaceutical Holdings Co., Ltd., Biofermin Pharmaceutical Co., Ltd., Mylan EPD G. K., Daiichi Sankyo Inc., Zeria Pharmaceutical Co., Ltd., and (2) those from which the authors have received research grant; AbbVie GK, GlaxoSmithKline K. K., Janssen Pharmaceutical K. K., EA Pharma Co., Ltd., Fujifilm Corporation, Mochida Pharmaceutical Co., Ltd., Aska Pharmaceutical Co., Ltd., Astellas Pharma Inc., Gilead Sciences, Inc., Biofermin Pharmaceutical Co., Ltd., Mylan EPD G. K., Tsumura and Co., Zespri International Limited, and (3) those from which the authors have received scholarship; Eisai Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Tobishima Kodomo Clinic, Takeda Pharmaceutical Company, Daiichi Sankyo Inc., AbbVie GK, Kyorin Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Bristol-Myers Squibb K. K., EA Pharma Co., Ltd., Mochida Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K. K., and (4) those from which the authors have received individually endowed chair; Ono Pharmaceutical Co., Ltd., Miyarisan Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Otsuka Pharmaceutical Factory, Inc., Fujifilm Medical Co., Ltd., Terumo Corporation, FANCL Corporation, Ohga Pharmacy, Abbott Japan LLC, and Muta Hospital.
Figures

References
-
- Schiller LR, Pardi DS, Sellin JH. Chronic diarrhea: diagnosis and management. Clin Gastroenterol Hepatol. 2017;15(2):182–93.e3. - PubMed
-
- Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, et al. . Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome foundation global study. Gastroenterology. 2021;160(1):99–114.e3. - PubMed
-
- Li H, Xing X, Yao L, Li M, Xun Y, Yan P, et al. . Assessment of the quality and content of clinical practice guidelines on irritable bowel syndrome using the AGREE II instrument. Digestion. 2020;101(4):355–65. - PubMed
-
- Yoshida M, Kinoshita Y, Watanabe M, Sugano K. JSGE Clinical Practice Guidelines 2014: standards, methods, and process of developing the guidelines. J Gastroenterol. 2015;50(1):4–10. - PubMed
-
- Qaseem A, Kansagara D, Lin JS, Mustafa RA, Wilt TJ; Clinical Guidelines Committee of the American College of Physicians, et al. . The development of clinical guidelines and guidance statements by the Clinical Guidelines Committee of the American College of Physicians: update of methods. Ann Intern Med. 2019;170(12):863–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

