Design of a Phase 3, Global, Multicenter, Randomized, Placebo-Controlled, Double-Blind Study of Nipocalimab in Pregnancies at Risk for Severe Hemolytic Disease of the Fetus and Newborn
- PMID: 39197469
- PMCID: PMC12020506
- DOI: 10.1055/a-2404-8089
Design of a Phase 3, Global, Multicenter, Randomized, Placebo-Controlled, Double-Blind Study of Nipocalimab in Pregnancies at Risk for Severe Hemolytic Disease of the Fetus and Newborn
Abstract
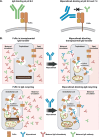
Nipocalimab is a neonatal fragment crystallizable (Fc) receptor (FcRn)-blocking monoclonal antibody that inhibits placental immunoglobulin G (IgG) transfer and lowers circulating maternal IgG levels. In an open-label, single-arm, phase 2 study, nipocalimab demonstrated evidence of safety and efficacy that support further investigation in a pivotal phase 3 trial of recurrent hemolytic disease of the fetus and newborn (HDFN). The phase 3 AZALEA study aims to evaluate the efficacy and safety of nipocalimab in a larger population at risk for severe HDFN, defined as HDFN associated with poor fetal outcomes or neonatal death.AZALEA is a multicenter, randomized, placebo-controlled, double-blind, phase 3 study enrolling alloimmunized pregnant individuals (N ≈ 120) at risk for severe HDFN based on obstetric history. Participants are randomized 2:1 to receive intravenous 45 mg/kg nipocalimab or placebo weekly from 13-16 to 35 weeks gestational age (GA). During the double-blind treatment period, participants receive standard-of-care weekly monitoring for fetal anemia until planned delivery at 37 to 38 weeks of GA. Postnatal follow-up periods are 24 weeks for maternal participants and 104 weeks for neonates/infants.The primary endpoint is the proportion of pregnancies that do not result in intrauterine transfusion (IUT), hydrops fetalis, or fetal loss/neonatal death from all causes. Key secondary endpoints include the severity of HDFN as measured by a composite HDFN severity index, the earliest time to occurrence of IUT or hydrops fetalis, the modified neonatal mortality and morbidity index in liveborn neonates, and the number of IUTs received. Other endpoints are safety, patient- and caregiver-reported outcomes, pharmacokinetics, pharmacodynamics (e.g., IgG, FcRn receptor occupancy), and immunogenicity of nipocalimab.AZALEA, the first placebo-controlled, randomized, multicenter, prospective trial in severe HDFN, is designed to evaluate the safety and efficacy of nipocalimab, a potential preventive and noninvasive intervention, in at-risk HDFN pregnancies. · Severe HDFN leads to poor fetal/neonatal outcomes.. · IUTs are associated with complications and fetal loss.. · Nipocalimab blocks IgG recycling and placental transfer.. · Nipocalimab reduces fetal anemia and IUTs in early-onset severe HDFN.. · The phase 3 AZALEA study evaluates nipocalimab in severe HDFN..
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
Y.K., P.A., E. Lam, J.H.L., L.E.L., R.M.N., V.O., S.S-K., M.L.T., J.Z., U.A., and W.S. are employees of Janssen and hold stock/stock options from Johnson & Johnson. E.J.T.V. serves as the principal investigator of the UNITY, CLARITY, and AZALEA studies in The Netherlands. E.T., E. Lopriore, and D.O. received consulting fees for membership of steering committees and advisory boards for clinical studies from Momenta Pharmaceuticals, Inc., and Janssen Pharmaceuticals, Inc. K.J.M. serves as the overall principal investigator for the phase 2 trial of nipocalimab (UNITY); received funding from Momenta Pharmaceuticals, Inc., paid on his behalf to the McGovern Medical School – UT Health; received funding from Janssen Pharmaceuticals, Inc., paid on his behalf to Dell Medical School at The University of Texas at Austin for a clinical trial on a monoclonal antibody for the treatment of HDFN; served on the steering committees and advisory boards for clinical studies for Momenta Pharmaceuticals, Inc., and Janssen Pharmaceuticals, Inc., but has not received funding for these activities; received royalty funding from UpToDate, Inc., for authorship of various chapters; received consulting fees from Health Management Associates, Inc., for consultation on the formation of fetal centers; received consulting fees from BillionToOne, Inc., paid on his behalf to Dell Medical School at The University of Texas at Austin; received honoraria from GLC Healthcare, Inc., for podcast content on HDFN; and serves as a nonpaid consultant for immunology at Janssen Pharmaceuticals, Inc.
Figures


References
-
- de Haas M, Thurik F F, Koelewijn J M, van der Schoot C E. Haemolytic disease of the fetus and newborn. Vox Sang. 2015;109(02):99–113. - PubMed
-
- Society for Maternal-Fetal Medicine (SMFM). Electronic address: pubs@smfm.org . Mari G, Norton M E, Stone J et al.Society for Maternal-Fetal Medicine (SMFM) Clinical Guideline #8: the fetus at risk for anemia–diagnosis and management. Am J Obstet Gynecol. 2015;212(06):697–710. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

