Structure-Activity Relationship of Antibody-Oligonucleotide Conjugates: Evaluating Bioconjugation Strategies for Antibody-siRNA Conjugates for Drug Development
- PMID: 39197831
- PMCID: PMC11403602
- DOI: 10.1021/acs.jmedchem.4c00802
Structure-Activity Relationship of Antibody-Oligonucleotide Conjugates: Evaluating Bioconjugation Strategies for Antibody-siRNA Conjugates for Drug Development
Abstract
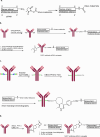
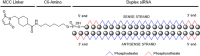
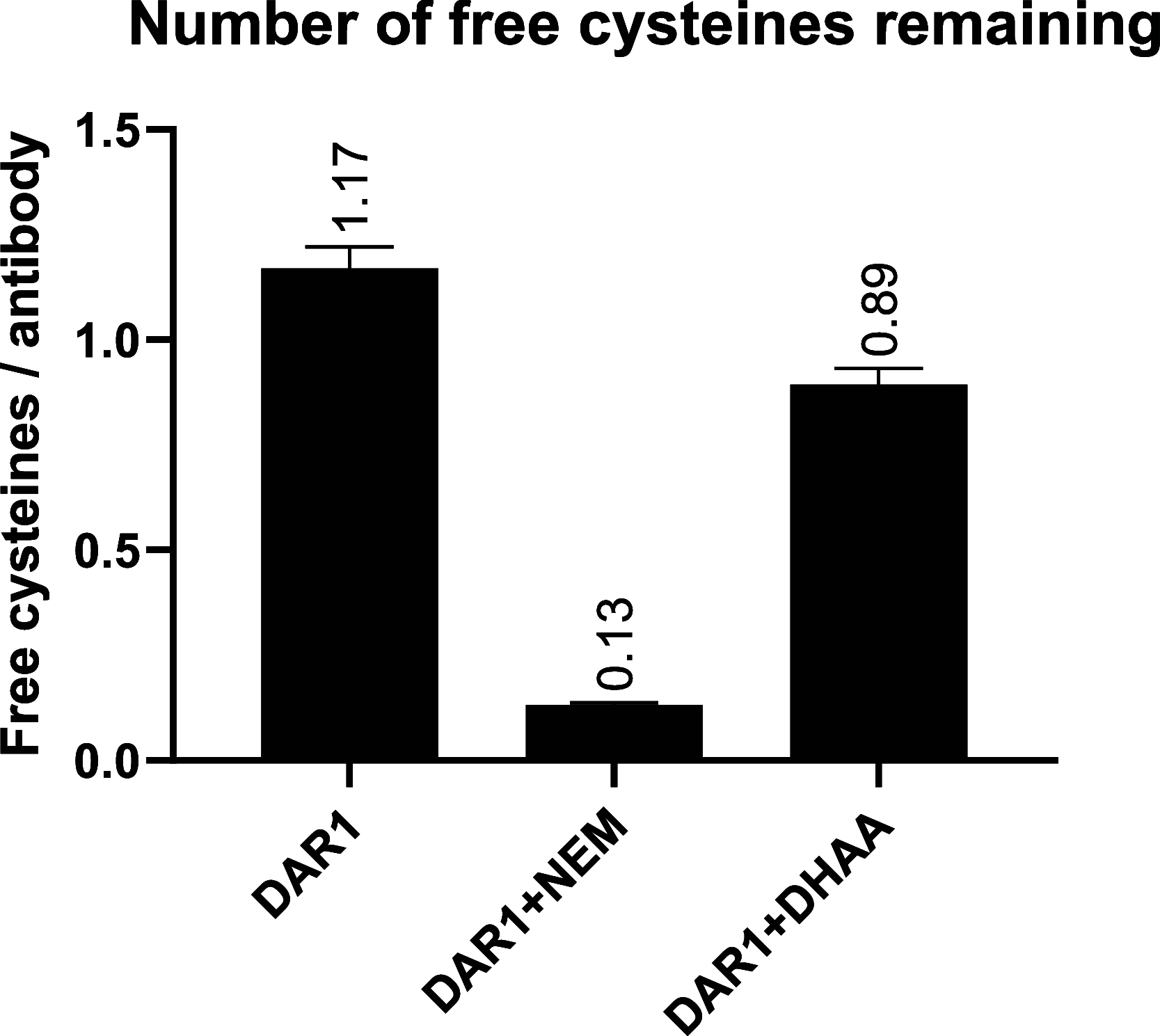
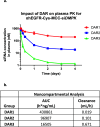
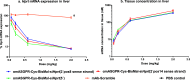
Antibody-oligonucleotide conjugates are a promising class of therapeutics for extrahepatic delivery of small interfering ribonucleic acids (siRNAs). These conjugates can be optimized for improved delivery and mRNA knockdown (KD) through understanding of structure-activity relationships. In this study, we systematically examined factors including antibody isotype, siRNA chemistry, linkers, conjugation chemistry, PEGylation, and drug-to-antibody ratios (DARs) for their impact on bioconjugation, pharmacokinetics (PK), siRNA delivery, and bioactivity. Conjugation site (cysteine, lysine, and Asn297 glycan) and DAR proved critical for optimal conjugate PK and siRNA delivery. SiRNA chemistry including 2' sugar modifications and positioning of phosphorothioates were found to be critical for delivery and duration of action. By utilizing cleavable and noncleavable linkers, we demonstrated the impact of linkers on PK and mRNA KD. To achieve optimal properties of antibody-siRNA conjugates, a careful selection of siRNA chemistry, DAR, conjugation sites, linkers, and antibody isotype is necessary.
Conflict of interest statement
The authors declare the following competing financial interest(s): At the time this research was conducted, all authors were employees of Avidity Biosciences and may have equity in the company.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

