Low-Frequency Oscillations in Mid-rostral Dorsolateral Prefrontal Cortex Support Response Inhibition
- PMID: 39197939
- PMCID: PMC11450526
- DOI: 10.1523/JNEUROSCI.0122-24.2024
Low-Frequency Oscillations in Mid-rostral Dorsolateral Prefrontal Cortex Support Response Inhibition
Abstract
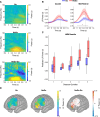
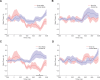
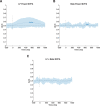
Executive control of movement enables inhibiting impulsive responses critical for successful navigation of the environment. Circuits mediating stop commands involve prefrontal and basal ganglia structures with fMRI evidence demonstrating increased activity during response inhibition in the dorsolateral prefrontal cortex (dlPFC)-often ascribed to maintaining task attentional demands. Using direct intraoperative cortical recordings in male and female human subjects, we investigated oscillatory dynamics along the rostral-caudal axis of dlPFC during a modified Go/No-go task, probing components of both proactive and reactive motor control. We assessed whether cognitive control is topographically organized along this axis and observed that low-frequency power increased prominently in mid-rostral dlPFC when inhibiting and delaying responses. These findings provide evidence for a key role for mid-rostral dlPFC low-frequency oscillations in sculpting motor control.
Keywords: cognitive control; dorsolateral prefrontal cortex; electrocorticography; local field potentials; response inhibition.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
