Altered lipid metabolism promoting cardiac fibrosis is mediated by CD34+ cell-derived FABP4+ fibroblasts
- PMID: 39198543
- PMCID: PMC11372182
- DOI: 10.1038/s12276-024-01309-9
Altered lipid metabolism promoting cardiac fibrosis is mediated by CD34+ cell-derived FABP4+ fibroblasts
Abstract
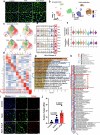
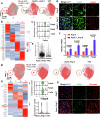
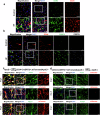
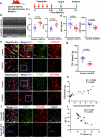
Hyperlipidemia and hypertension might play a role in cardiac fibrosis, in which a heterogeneous population of fibroblasts seems important. However, it is unknown whether CD34+ progenitor cells are involved in the pathogenesis of heart fibrosis. This study aimed to explore the mechanism of CD34+ cell differentiation in cardiac fibrosis during hyperlipidemia. Through the analysis of transcriptomes from 50,870 single cells extracted from mouse hearts and 76,851 single cells from human hearts, we have effectively demonstrated the evolving cellular landscape throughout cardiac fibrosis. Disturbances in lipid metabolism can accelerate the development of fibrosis. Through the integration of bone marrow transplantation models and lineage tracing, our study showed that hyperlipidemia can expedite the differentiation of non-bone marrow-derived CD34+ cells into fibroblasts, particularly FABP4+ fibroblasts, in response to angiotensin II. Interestingly, the partial depletion of CD34+ cells led to a notable reduction in triglycerides in the heart, mitigated fibrosis, and improved cardiac function. Furthermore, immunostaining of human heart tissue revealed colocalization of CD34+ cells and fibroblasts. Mechanistically, our investigation of single-cell RNA sequencing data through pseudotime analysis combined with in vitro cellular studies revealed the crucial role of the PPARγ/Akt/Gsk3β pathway in orchestrating the differentiation of CD34+ cells into FABP4+ fibroblasts. Through our study, we generated valuable insights into the cellular landscape of CD34+ cell-derived cells in the hypertrophic heart with hyperlipidemia, indicating that the differentiation of non-bone marrow-derived CD34+ cells into FABP4+ fibroblasts during this process accelerates lipid accumulation and promotes heart failure via the PPARγ/Akt/Gsk3β pathway.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Tanai, E. & Frantz, S. Pathophysiology of heart failure. Compr Physiol. 6, 187–214 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

