Translating biological insights into improved management of endometrial cancer
- PMID: 39198622
- PMCID: PMC12084113
- DOI: 10.1038/s41571-024-00934-7
Translating biological insights into improved management of endometrial cancer
Abstract
Endometrial cancer (EC) is the most common gynaecological cancer among women in high-income countries, with both incidence and mortality continuing to increase. The complexity of the management of patients with EC has evolved with greater comprehension of the underlying biology and heterogeneity of this disease. With a growing number of novel therapeutic agents available, emerging treatment regimens seem to have the potential to help to address the concerning trends in EC-related mortality. In this Review, we describe the epidemiology, histopathology and molecular classification of EC as well as the role of the new (2023) International Federation of Gynecologists and Obstetricians (FIGO) staging model. Furthermore, we provide an overview of disease management in the first-line and recurrent disease settings. With increasing use of molecular profiling and updates in treatment paradigms, we also summarize new developments in this rapidly changing treatment landscape.
© 2024. Springer Nature Limited.
Conflict of interest statement
Competing interests
Jeffrey A. How, Barrett Lawson, Ann H. Klopp, and Karen H. Lu report no competing interests.
Amir A. Jazaeri reports personal fees from Gerson Lehrman Group, Guidepoint, Iovance advisory board meeting, NuProbe, Simcere, PACT Pharma, Genentech-Roche, Eisai, Agenus, and Macrogenics. He also reports grants from AstraZeneca, Bristol Myers Squibb, Iovance, Aravive, Pfizer, Immatics US, Eli Lilly, Merck, and stock/stock options from AvengeBio outside submitted work.
Shannon N. Westin reports grants from NIH, GOG Foundation, Cotinga Pharmaceuticals, Bayer, and ArQule during the conduct of the study. She also reports grants and personal fees from AstraZeneca, Clovis Oncology, GlaxoSmithKline/Tesaro, Roche/Genentech, and Novartis. She reports personal fees from Merck, Pfizer, Eisai, personal CIrculogene, Zentalis, and Agenus outside the submitted work. NDF reports personal fees from Tesaro, Pfizer, Bristol Myers Squibb, and GlaxoSmithKline outside the submitted work.
Pamela T Soliman reports clinical trial support or research grants to the institution from Merck, Novartis, Incyte, GSK as well as consulting fees from Aadi, GSK, Essau.
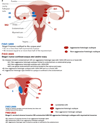
Figures




References
-
- Ferlay J EM, Lam F, Laversanne M, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F Corpus uteri fact sheet, <https://gco.iarc.who.int/today > (2024).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

