Tuberculosis in otherwise healthy adults with inherited TNF deficiency
- PMID: 39198650
- PMCID: PMC11390478
- DOI: 10.1038/s41586-024-07866-3
Tuberculosis in otherwise healthy adults with inherited TNF deficiency
Abstract
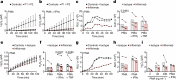
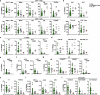
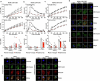
Severe defects in human IFNγ immunity predispose individuals to both Bacillus Calmette-Guérin disease and tuberculosis, whereas milder defects predispose only to tuberculosis1. Here we report two adults with recurrent pulmonary tuberculosis who are homozygous for a private loss-of-function TNF variant. Neither has any other clinical phenotype and both mount normal clinical and biological inflammatory responses. Their leukocytes, including monocytes and monocyte-derived macrophages (MDMs) do not produce TNF, even after stimulation with IFNγ. Blood leukocyte subset development is normal in these patients. However, an impairment in the respiratory burst was observed in granulocyte-macrophage colony-stimulating factor (GM-CSF)-matured MDMs and alveolar macrophage-like (AML) cells2 from both patients with TNF deficiency, TNF- or TNFR1-deficient induced pluripotent stem (iPS)-cell-derived GM-CSF-matured macrophages, and healthy control MDMs and AML cells differentiated with TNF blockers in vitro, and in lung macrophages treated with TNF blockers ex vivo. The stimulation of TNF-deficient iPS-cell-derived macrophages with TNF rescued the respiratory burst. These findings contrast with those for patients with inherited complete deficiency of the respiratory burst across all phagocytes, who are prone to multiple infections, including both Bacillus Calmette-Guérin disease and tuberculosis3. Human TNF is required for respiratory-burst-dependent immunity to Mycobacterium tuberculosis in macrophages but is surprisingly redundant otherwise, including for inflammation and immunity to weakly virulent mycobacteria and many other infectious agents.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures










References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

