Health and economic impacts of Lassa vaccination campaigns in West Africa
- PMID: 39198710
- PMCID: PMC11645265
- DOI: 10.1038/s41591-024-03232-y
Health and economic impacts of Lassa vaccination campaigns in West Africa
Abstract
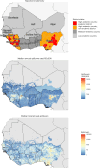
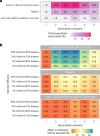
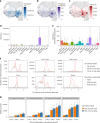
Lassa fever is a zoonotic disease identified by the World Health Organization (WHO) as having pandemic potential. This study estimates the health-economic burden of Lassa fever throughout West Africa and projects impacts of a series of vaccination campaigns. We also model the emergence of 'Lassa-X'-a hypothetical pandemic Lassa virus variant-and project impacts of achieving 100 Days Mission vaccination targets. Our model predicted 2.7 million (95% uncertainty interval: 2.1-3.4 million) Lassa virus infections annually, resulting over 10 years in 2.0 million (793,800-3.9 million) disability-adjusted life years (DALYs). The most effective vaccination strategy was a population-wide preventive campaign primarily targeting WHO-classified 'endemic' districts. Under conservative vaccine efficacy assumptions, this campaign averted $20.1 million ($8.2-$39.0 million) in lost DALY value and $128.2 million ($67.2-$231.9 million) in societal costs (2021 international dollars ($)). Reactive vaccination in response to local outbreaks averted just one-tenth the health-economic burden of preventive campaigns. In the event of Lassa-X emerging, spreading throughout West Africa and causing approximately 1.2 million DALYs within 2 years, 100 Days Mission vaccination averted 22% of DALYs given a vaccine 70% effective against disease and 74% of DALYs given a vaccine 70% effective against both infection and disease. These findings suggest how vaccination could alleviate Lassa fever's burden and assist in pandemic preparedness.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

Update of
-
Health and economic impacts of Lassa vaccination campaigns in West Africa.medRxiv [Preprint]. 2024 Jun 30:2024.02.26.24303394. doi: 10.1101/2024.02.26.24303394. medRxiv. 2024. Update in: Nat Med. 2024 Dec;30(12):3568-3577. doi: 10.1038/s41591-024-03232-y. PMID: 38978680 Free PMC article. Updated. Preprint.
References
-
- World Health Organization. Lassa fever. https://www.who.int/health-topics/lassa-fever#tab=tab_1 (2023).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

