The evolving genetic landscape of telomere biology disorder dyskeratosis congenita
- PMID: 39198715
- PMCID: PMC11473520
- DOI: 10.1038/s44321-024-00118-x
The evolving genetic landscape of telomere biology disorder dyskeratosis congenita
Abstract
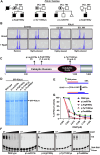
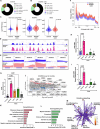
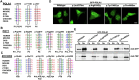
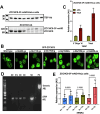
Dyskeratosis congenita (DC) is a rare inherited bone marrow failure syndrome, caused by genetic mutations that principally affect telomere biology. Approximately 35% of cases remain uncharacterised at the genetic level. To explore the genetic landscape, we conducted genetic studies on a large collection of clinically diagnosed cases of DC as well as cases exhibiting features resembling DC, referred to as 'DC-like' (DCL). This led us to identify several novel pathogenic variants within known genetic loci and in the novel X-linked gene, POLA1. In addition, we have also identified several novel variants in POT1 and ZCCHC8 in multiple cases from different families expanding the allelic series of DC and DCL phenotypes. Functional characterisation of novel POLA1 and POT1 variants, revealed pathogenic effects on protein-protein interactions with primase, CTC1-STN1-TEN1 (CST) and shelterin subunit complexes, that are critical for telomere maintenance. ZCCHC8 variants demonstrated ZCCHC8 deficiency and signs of pervasive transcription, triggering inflammation in patients' blood. In conclusion, our studies expand the current genetic architecture and broaden our understanding of disease mechanisms underlying DC and DCL disorders.
Keywords: POLA1; Dyskeratosis Congenita; Telomeres; ncRNAs.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Anderson BH, Kasher PR, Mayer J, Szynkiewicz M, Jenkinson EM, Bhaskar SS, Urquhart JE, Daly SB, Dickerson JE, O’Sullivan J et al (2012) Mutations in CTC1, encoding conserved telomere maintenance component 1, cause Coats plus. Nat Genet 44:338–342. 10.1038/ng.1084 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

