Vaginal and endometrial microbiome dysbiosis associated with adverse embryo transfer outcomes
- PMID: 39198832
- PMCID: PMC11351450
- DOI: 10.1186/s12958-024-01274-y
Vaginal and endometrial microbiome dysbiosis associated with adverse embryo transfer outcomes
Abstract
Background: Assisted reproductive technology (ART) is the most effective method to treat infertility and the pathogenesis of implantation failure after in vitro fertilization-embryo transfer (IVF-ET) is a challenging filed in infertility. Microbes in the female reproductive tract are considered to be associated with gynecological and obstetric diseases. However, its effects on embryo implantation failure are unsured.
Purpose: This study aimed to investigate reproductive tract dysbiosis, identify different bacteria in reproductive tract as potential biomarkers of embryo implantation failure and demonstrate the pathogenesis through metabolites analysis.
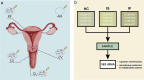
Methods: We compared the data from 16S rRNA gene and metagenome in reproductive tracts through QIIME2 and HUMAnN2 by the times of embryo implantation failure on 239 infertile patients and 17 healthy women.
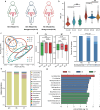
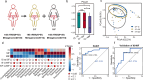
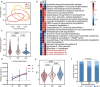
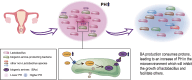
Results: Our study revealed a strong positive correlation between Lactobacillus abundance and embryo implantation success (IS) after IVF-ET. The microbial community composition and structure in reproductive tract showed substantially difference between the embryo implantation failure (IF) and healthy control. Moreover, we established a diagnostic model through receiver operating characteristic (ROC) with 0.913 area under curve (AUC) in IS and multiple implantation failures (MIF), verified its effectiveness with an AUC = 0.784 demonstrating microbial community alterations could efficiently discriminate MIF patients. Metagenome functional analyses of vaginal samples from another independent infertile patients after IVF-ET revealed the L-lysine synthesis pathway enriched in IF patients, along with ascended vaginal pH and decreased Lactobacillus abundance.
Conclusions: This study clarifies several independent relationships of bacteria in vagina and endometrial fluid on embryo implantation failure and undoubtedly broadens the understanding about female reproductive health.
Keywords: Lactobacillus; Diagnostic model; Implantation failure; In vitro fertilization-embryo transfer; Infertility; Microbiome; Reproductive tract.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

