Empagliflozin and left atrial function in patients with type 2 diabetes mellitus and coronary artery disease: insight from the EMPA-HEART CardioLink-6 randomized clinical trial
- PMID: 39198860
- PMCID: PMC11360285
- DOI: 10.1186/s12933-024-02344-6
Empagliflozin and left atrial function in patients with type 2 diabetes mellitus and coronary artery disease: insight from the EMPA-HEART CardioLink-6 randomized clinical trial
Abstract
Background: Sodium-glucose cotransporter-2 (SGLT2) inhibitors have demonstrated reduction in heart failure outcomes in patients with type 2 diabetes mellitus, although the exact mechanism of benefit remains unclear. Alteration in left atrial (LA) function due to chronic pressure or volume overload is a hallmark of heart failure.
Objective: To evaluate the effect of the SGLT2 inhibitor empagliflozin on LA volume and function.
Methods: 90 patients with coronary artery disease and type 2 diabetes (T2DM) were randomized to empagliflozin (n = 44) or placebo (n = 46), and underwent cardiac magnetic resonance (CMR) imaging at baseline and after 6 months. The main outcome was change in LA volume; LA function, including active and passive components, was also measured by a blinded reader.
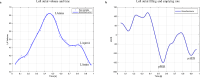
Results: At baseline, there was no significant difference in LA volumes between the empagliflozin (indexed maximum LA volume 26.4 ± 8.4mL/m2, minimum LA volume 11.1 ± 5.7mL/m2) and placebo (indexed maximum LA volume 28.7 ± 8.2mL/m2, minimum LA volume 12.6 ± 5.0mL/m2) groups. After 6 months, changes in LA volumes did not differ with adjusted difference (empagliflozin minus placebo): 0.99 mL/m2 (95% CI: -1.7 to 3.7 mL/m2; p = 0.47) for indexed maximum LA volume, and 0.87 mL/m2 (95% CI: -0.9 to 2.6 mL/m2; p = 0.32) for indexed minimum LA volume. Changes in total LA emptying fraction were also similar, with between-group adjusted mean difference - 0.01 (95% CI: -0.05 to 0.03, p = 0.59).
Conclusion: SGLT2 inhibition with empagliflozin for 6 months did not have a significant impact on LA volume and function in patients with T2DM and coronary artery disease. (Effects of Empagliflozin on Cardiac Structure in Patients with Type 2 Diabetes [EMPA-HEART]; NCT02998970).
Keywords: Cardiac MRI; Diabetes; Empagliflozin; Left atrial function.
© 2024. The Author(s).
Conflict of interest statement
Marina Pourafkari, None. Kim A. Connelly, Dr Connelly is listed as an inventor on a patent application by Boehringer Ingelheim on the use of dipeptidyl peptidase-4 inhibitors in heart failure; and reports receiving research grants to his institution from AstraZeneca, Servier and Boehringer Ingelheim; support for travel to scientific meetings from Boehringer Ingelheim and honoraria for speaking engagements and ad hoc participation in advisory boards from Servier, Merck, Eli Lilly, AstraZeneca, Boehringer Ingelheim, Ferring, Novo Nordisk, Novartis and Janssen. Subodh Verma, Dr Verma holds a Tier 1 Canada Research Chair in Cardiovascular Surgery and reports receiving research grants and/or speaking honoraria from Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Canadian Medical and Surgical Knowledge Translation Research Group, Eli Lilly, HLS Therapeutics, Janssen, Novartis, Novo Nordisk, Pfizer, PhaseBio, Sanofi, and S&L Solutions; he is the President of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not-for-profit physician organization. C. David Mazer, Dr Mazer is supported by a Merit Award from the University of Toronto Department of Anesthesiology and Pain Medicine, holds the Cara Phelan Chair in Critical Care at St. Michael’s Hospital-Unity Health Toronto, and reports advisory board honoraria/consulting fees from Amgen, AstraZeneca, BioAge, Boehringer Ingelheim, and PhaseBio and DSMB stipends from Beth Israel Deaconess Medical Center, Cerus, and Takeda. Hwee Teoh, Dr. Teoh reports personal fees from the Canadian Medical and Surgical Knowledge Translation Research Group. Adrian Quan, None. Shaun G. Goodman, Research grant support (e.g., steering committee or data and safety monitoring committee) and/or speaker/consulting honoraria (e.g., advisory boards) from: Amgen, Anthos Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, CYTE Ltd., Daiichi-Sankyo/American Regent, Eli Lilly, Esperion, Ferring Pharmaceuticals, HLS Therapeutics, Idorsia, JAMP Pharma, Merck, Novartis, Novo Nordisk A/C, Pendopharm/Pharmascience, Pfizer, Regeneron, Sanofi, Servier, Tolmar Pharmaceuticals, Valeo Pharma; and salary support/honoraria from the Canadian Heart Failure Society, Canadian Heart Research Centre and MD Primer, Canadian VIGOUR Centre, Cleveland Clinic Coordinating Centre for Clinical Research, Duke Clinical Research Institute, Jewish General Hospital\ CIUSSS Centre-Ouest-de-l’Ile-de-Montreal, New York University Clinical Coordinating Centre, PERFUSE Research Institute, Peter Munk Cardiac Centre Clinical Trials and Translation Unit, TIMI Study Group (Brigham Health). Archana Rai, None. Ming Yen Ng. Dr Ng reports receiving educational grants from Bayer, GE Healthcare, Circle Cardiovascular Imaging, and TeraRecon. Received speakers fees from Boerhinger Ingelheim, Bayer, GE Healthcare and Circle Cardiovascular Imaging. Djeven P. Deva, None. Piero Triverio, None. Laura Jiminez Juan, None. Andrew T. Yan, None. Yin Ge, None.
Figures

References
-
- Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, et al. 2022 AHA/ACC/HFSA Guideline for the management of Heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice guidelines. J Am Coll Cardiol. 2022;79(17):e263–421. 10.1016/j.jacc.2021.12.012 - DOI - PubMed
-
- de Boer IH, Khunti K, Sadusky T, Tuttle KR, Neumiller JJ, Rhee CM, Rosas SE, Rossing P, Bakris G. Diabetes management in chronic kidney disease: a Consensus Report by the American Diabetes Association (ADA) and kidney disease: improving global outcomes (KDIGO). Diabetes Care. 2022;45(12):3075–90. 10.2337/dci22-0027 - DOI - PMC - PubMed
-
- Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, Zuo F, Quan A, Farkouh ME, Fitchett DH, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation. 2019;140(21):1693–702. 10.1161/CIRCULATIONAHA.119.042375 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

