The Impairment of Endothelial Autophagy Accelerates Renal Senescence by Ferroptosis and NLRP3 Inflammasome Signaling Pathways with the Disruption of Endothelial Barrier
- PMID: 39199133
- PMCID: PMC11351978
- DOI: 10.3390/antiox13080886
The Impairment of Endothelial Autophagy Accelerates Renal Senescence by Ferroptosis and NLRP3 Inflammasome Signaling Pathways with the Disruption of Endothelial Barrier
Abstract
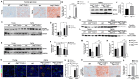
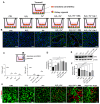
Autophagy is a cellular process that degrades damaged cytoplasmic components and regulates cell death. The homeostasis of endothelial cells (ECs) is crucial for the preservation of glomerular structure and function in aging. Here, we investigated the precise mechanisms of endothelial autophagy in renal aging. The genetic deletion of Atg7 in the ECs of Atg7flox/flox;Tie2-Cre mice accelerated aging-related glomerulopathy and tubulointerstitial fibrosis. The EC-specific Atg7 deletion in aging mice induced the detachment of EC with the disruption of glomerular basement membrane (GBM) assembly and increased podocyte loss resulting in microalbuminuria. A Transwell co-culture system of ECs and kidney organoids showed that the iron and oxidative stress induce the disruption of the endothelial barrier and increase vascular permeability, which was accelerated by the inhibition of autophagy. This resulted in the leakage of iron through the endothelial barrier into kidney organoids and increased oxidative stress, which led to ferroptotic cell death. The ferritin accumulation was increased in the kidneys of the EC-specific Atg7-deficient aging mice and upregulated the NLRP3 inflammasome signaling pathway. The pharmacologic inhibition of ferroptosis with liproxstatin-1 recovered the disrupted endothelial barrier and reversed the decreased expression of GPX4, as well as NLRP3 and IL-1β, in endothelial autophagy-deficient aged mice, which attenuated aging-related renal injury including the apoptosis of renal cells, abnormal structures of GBM, and tubulointerstitial fibrosis. Our data showed that endothelial autophagy is essential for the maintenance of the endothelial barrier during renal aging and the impairment of endothelial autophagy accelerates renal senescence by ferroptosis and NLRP3 inflammasome signaling pathways. These processes may be attractive therapeutic targets to reduce cellular injury from renal aging.
Keywords: aging; autophagy; kidney; liproxstatin-1.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Hong Y.A., Ban T.H., Kang C.-Y., Hwang S.D., Choi S.R., Lee H., Jung H.-Y., Kim K., Kwon Y.E., Kim S.H. Trends in epidemiologic characteristics of end-stage renal disease from 2019 Korean Renal Data System (KORDS) Kidney Res. Clin. Pract. 2021;40:52. doi: 10.23876/j.krcp.20.202. - DOI - PMC - PubMed
-
- Kim H., Jeong S.A., Kim K.M., Hwang S.D., Choi S.R., Lee H., Kim J.H., Kim S.H., Kim T.H., Koo H.-S. Trends in clinical outcomes of older hemodialysis patients: Data from the 2023 Korean Renal Data System (KORDS) Kidney Res. Clin. Pract. 2024;43:263. doi: 10.23876/j.krcp.23.267. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

