Targeting Circadian Protein Rev-erbα to Alleviate Inflammation, Oxidative Stress, and Enhance Functional Recovery Following Brain Trauma
- PMID: 39199147
- PMCID: PMC11351136
- DOI: 10.3390/antiox13080901
Targeting Circadian Protein Rev-erbα to Alleviate Inflammation, Oxidative Stress, and Enhance Functional Recovery Following Brain Trauma
Abstract
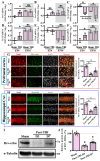
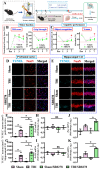
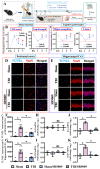
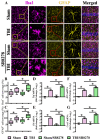
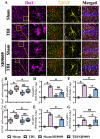
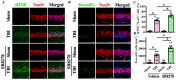
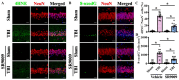
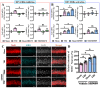
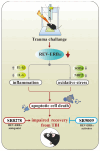
Traumatic brain injury (TBI) is a significant cause of morbidity and mortality worldwide, and its pathophysiology is characterized by oxidative stress and inflammation. Despite extensive research, effective treatments for TBI remain elusive. Recent studies highlighted the critical interplay between TBI and circadian rhythms, but the detailed regulation remains largely unknown. Motivated by the observed sustained decrease in Rev-erbα after TBI, we aimed to understand the critical role of Rev-erbα in the pathophysiology of TBI and determine its feasibility as a therapeutic target. Using a mouse model of TBI, we observed that TBI significantly downregulates Rev-erbα levels, exacerbating inflammatory and oxidative stress pathways. The regulation of Rev-erbα with either the pharmacological activator or inhibitor bidirectionally modulated inflammatory and oxidative events, which in turn influenced neurobehavioral outcomes, highlighting the protein's protective role. Mechanistically, Rev-erbα influences the expression of key oxidative stress and inflammatory regulatory genes. A reduction in Rev-erbα following TBI likely contributes to increased oxidative damage and inflammation, creating a detrimental environment for neuronal survival and recovery which could be reversed via the pharmacological activation of Rev-erbα. Our findings highlight the therapeutic potential of targeting Rev-erbα to mitigate TBI-induced damage and improve outcomes, especially in TBI-susceptible populations with disrupted circadian regulation.
Keywords: NR1D1; SR8278; SR9009; inflammation; neuronal cell death; oxidative stress; traumatic brain injury.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Maas A.I.R., Menon D.K., Adelson P.D., Andelic N., Bell M.J., Belli A., Bragge P., Brazinova A., Büki A., Chesnut R.M., et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. doi: 10.1016/S1474-4422(17)30371-X. - DOI - PubMed
-
- Howe E.I., Andelic N., Fure S.C.R., Røe C., Søberg H.L., Hellstrøm T., Spjelkavik Ø., Enehaug H., Lu J., Ugelstad H., et al. Cost-effectiveness analysis of combined cognitive and vocational rehabilitation in patients with mild-to-moderate TBI: Results from a randomized controlled trial. BMC Health Serv. Res. 2022;22:185. doi: 10.1186/s12913-022-07585-3. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

