Oxidative Stress and Mitochondria Are Involved in Anaphylaxis and Mast Cell Degranulation: A Systematic Review
- PMID: 39199166
- PMCID: PMC11352116
- DOI: 10.3390/antiox13080920
Oxidative Stress and Mitochondria Are Involved in Anaphylaxis and Mast Cell Degranulation: A Systematic Review
Abstract
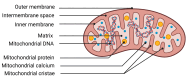
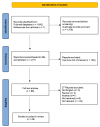
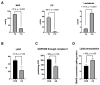
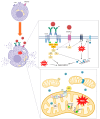
Anaphylaxis, an allergic reaction caused by the massive release of active mediators, can lead to anaphylactic shock (AS), the most severe and potentially life-threatening form of anaphylactic reaction. Nevertheless, understanding of its pathophysiology to support new therapies still needs to be improved. We performed a systematic review, assessing the role and the complex cellular interplay of mitochondria and oxidative stress during anaphylaxis, mast cell metabolism and degranulation. After presenting the main characteristics of anaphylaxis, the oxidant/antioxidant balance and mitochondrial functions, we focused this review on the involvement of mitochondria and oxidative stress in anaphylaxis. Then, we discussed the role of oxidative stress and mitochondria following mast cell stimulation by allergens, leading to degranulation, in order to further elucidate mechanistic pathways. Finally, we considered potential therapeutic interventions implementing these findings for the treatment of anaphylaxis. Experimental studies evaluated mainly cardiomyocyte metabolism during AS. Cardiac dysfunction was associated with left ventricle mitochondrial impairment and lipid peroxidation. Studies evaluating in vitro mast cell degranulation, following Immunoglobulin E (IgE) or non-IgE stimulation, revealed that mitochondrial respiratory complex integrity and membrane potential are crucial for mast cell degranulation. Antigen stimulation raises reactive oxygen species (ROS) production from nicotinamide adenine dinucleotide phosphate (NADPH) oxidases and mitochondria, leading to mast cell degranulation. Moreover, mast cell activation involved mitochondrial morphological changes and mitochondrial translocation to the cell surface near exocytosis sites. Interestingly, antioxidant administration reduced degranulation by lowering ROS levels. Altogether, these results highlight the crucial role of oxidative stress and mitochondria during anaphylaxis and mast cell degranulation. New therapeutics against anaphylaxis should probably target oxidative stress and mitochondria, in order to decrease anaphylaxis-induced systemic and major organ deleterious effects.
Keywords: anaphylactic shock; anaphylaxis; antioxidant; mast cells degranulation; mitochondria; oxidative stress; reactive oxygen species (ROS).
Conflict of interest statement
There are no conflicts of interest.
Figures


References
-
- Guerci P., Tacquard C., Chenard L., Millard D., Soufir L., Malinovsky J.-M., Garot M., Lalot J.-M., Besch G., Louis G., et al. Epidemiology and Outcome of Patients Admitted to Intensive Care after Anaphylaxis in France: A Retrospective Multicentre Study. Br. J. Anaesth. 2020;125:1025–1033. doi: 10.1016/j.bja.2020.08.024. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

