Photobiomodulation Mitigates PM2.5-Exacerbated Pathologies in a Mouse Model of Allergic Asthma
- PMID: 39199248
- PMCID: PMC11351905
- DOI: 10.3390/antiox13081003
Photobiomodulation Mitigates PM2.5-Exacerbated Pathologies in a Mouse Model of Allergic Asthma
Abstract
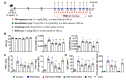
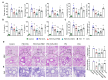
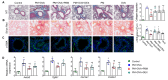
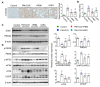
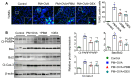
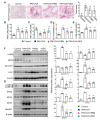
Exposure to particulate matter (PM), especially PM2.5, is known to exacerbate asthma, posing a significant public health risk. This study investigated the asthma-reducing effects of photobiomodulation (PBM) in a mice model mimicking allergic airway inflammation exacerbated by PM2.5 exposure. The mice received sensitization with ovalbumin (OVA) and were subsequently treated with PM2.5 at a dose of 0.1 mg/kg every 3 days, for 9 times over 3 weeks during the challenge. PBM, using a 610 nm wavelength LED, was applied at 1.7 mW/cm2 to the respiratory tract via direct skin contact for 20 min daily for 19 days. Results showed that PBM significantly reduced airway hyperresponsiveness, plasma immunoglobulin E (IgE) and OVA-specific IgE, airway inflammation, T-helper type 2 cytokine, histamine and tryptase in bronchoalveolar lavage fluid (BALF), and goblet cell hyperplasia in PM2.5-exposed asthmatic mice. Moreover, PBM alleviated subepithelial fibrosis by reducing collagen deposition, airway smooth muscle mass, and expression of fibrosis-related genes. It mitigated reactive oxygen species generation, oxidative stress, endoplasmic reticulum stress, apoptotic cell death, ferroptosis, and modulated autophagic signals in the asthmatic mice exposed to PM2.5. These findings suggest that PBM could be a promising intervention for PM2.5-induced respiratory complications in patients with allergic asthma.
Keywords: asthma; ferroptosis; oxidative stress; particulate matter (PM2.5); photobiomodulation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Hazlehurst M.F., Carroll K.N., Loftus C.T., Szpiro A.A., Moore P.E., Kaufman J.D., Kirwa K., LeWinn K.Z., Bush N.R., Sathyanarayana S. Maternal exposure to PM2. 5 during pregnancy and asthma risk in early childhood: Consideration of phases of fetal lung development. Environ. Epidemiol. 2021;5:e130. doi: 10.1097/EE9.0000000000000130. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

