Lysophosphatidylcholine Impairs the Mitochondria Homeostasis Leading to Trophoblast Dysfunction in Gestational Diabetes Mellitus
- PMID: 39199251
- PMCID: PMC11351454
- DOI: 10.3390/antiox13081007
Lysophosphatidylcholine Impairs the Mitochondria Homeostasis Leading to Trophoblast Dysfunction in Gestational Diabetes Mellitus
Abstract
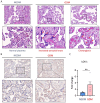
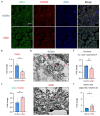
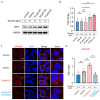
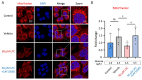
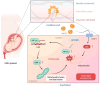
Gestational diabetes mellitus (GDM) is a common pregnancy disorder associated with an increased risk of pre-eclampsia and macrosomia. Recent research has shown that the buildup of excess lipids within the placental trophoblast impairs mitochondrial function. However, the exact lipids that impact the placental trophoblast and the underlying mechanism remain unclear. GDM cases and healthy controls were recruited at Kaohsiung Medical University Hospital. The placenta and cord blood were taken during birth. Confocal and electron microscopy were utilized to examine the morphology of the placenta and mitochondria. We determined the lipid composition using liquid chromatography-mass spectrometry in data-independent analysis mode (LC/MSE). In vitro studies were carried out on choriocarcinoma cells (JEG3) to investigate the mechanism of trophoblast mitochondrial dysfunction. Results showed that the GDM placenta was distinguished by increased syncytial knots, chorangiosis, lectin-like oxidized low-density lipoprotein (LDL) receptor-1 (LOX-1) overexpression, and mitochondrial dysfunction. Lysophosphatidylcholine (LPC) 16:0 was significantly elevated in the cord blood LDL of GDM patients. In vitro, we demonstrated that LPC dose-dependently disrupts mitochondrial function by increasing reactive oxygen species (ROS) levels and HIF-1α signaling. In conclusion, highly elevated LPC in cord blood plays a pivotal role in GDM, contributing to trophoblast impairment and pregnancy complications.
Keywords: gestational diabetes mellitus (GDM); hypoxia-induced factor-1 alpha (HIF-1α); lysophosphatidylcholine (LPC); mitochondrial dysfunction; placenta; trophoblast.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Quintanilla Rodriguez B.S., Mahdy H. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. Gestational Diabetes. - PubMed
-
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Metzger B.E., Gabbe S.G., Persson B., Buchanan T.A., Catalano P.A., Damm P., Dyer A.R., Leiva A., Hod M., et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. - DOI - PMC - PubMed
-
- HAPO Study Cooperative Research Group. Metzger B.E., Lowe L.P., Dyer A.R., Trimble E.R., Chaovarindr U., Coustan D.R., Hadden D.R., McCance D.R., Hod M., et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008;358:1991–2002. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

