The Effects of Hypoxia on the Immune-Metabolic Interplay in Liver Cancer
- PMID: 39199411
- PMCID: PMC11352590
- DOI: 10.3390/biom14081024
The Effects of Hypoxia on the Immune-Metabolic Interplay in Liver Cancer
Abstract
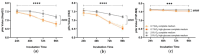
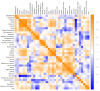
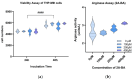
M2-like macrophages promote tumor growth and cancer immune evasion. This study used an in vitro model to investigate how hypoxia and tumor metabolism affect macrophage polarization. Liver cancer cells (HepG2 and VX2) and macrophages (THP1) were cultured under hypoxic (0.1% O2) and normoxic (21% O2) conditions with varying glucose levels (2 g/L or 4.5 g/L). Viability assays and extracellular pH (pHe) measurements were conducted over 96 hours. Macrophages were exposed to the tumor-conditioned medium (TCM) from the cancer cells, and polarization was assessed using arginase and nitrite assays. GC-MS-based metabolic profiling quantified TCM meta-bolites and correlated them with M2 polarization. The results showed that pHe in TCMs decreased more under hypoxia than normoxia (p < 0.0001), independent of glucose levels. The arginase assay showed hypoxia significantly induced the M2 polarization of macrophages (control group: p = 0.0120,0.1%VX2-TCM group: p = 0.0149, 0.1%HepG2-TCM group: p < 0.0001, 0.1%VX2-TCMHG group: p = 0.0001, and 0.1%HepG2-TCMHG group: p < 0.0001). TCMs also induced M2 polarization under normoxic conditions, but the strongest M2 polarization occurred when both tumor cells and macrophages were incubated under hypoxia with high glucose levels. Metabolomics revealed that several metabolites, particularly lactate, were correlated with hypoxia and M2 polarization. Under normoxia, elevated 2-amino-butanoic acid (2A-BA) strongly correlated with M2 polarization. These findings suggest that targeting tumor hypoxia could mitigate immune evasion in liver tumors. Lactate drives acidity in hypoxic tumors, while 2A-BA could be a therapeutic target for overcoming immunosuppression in normoxic conditions.
Keywords: 2-amino-butanoic acid (2A-BA); GC-MS-based metabolic profiling; M2-like polarization; hepatocellular carcinoma (HCC); hypoxia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

