Liver X Receptors Enhance Epithelial to Mesenchymal Transition in Metastatic Prostate Cancer Cells
- PMID: 39199549
- PMCID: PMC11353074
- DOI: 10.3390/cancers16162776
Liver X Receptors Enhance Epithelial to Mesenchymal Transition in Metastatic Prostate Cancer Cells
Abstract
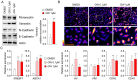
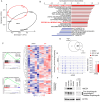
Prostate cancer (PCa) is one of the most common cancers in men. Metastasis is the leading cause of death in prostate cancer patients. One of the crucial processes involved in metastatic spread is the "epithelial-mesenchymal transition" (EMT), which allows cells to acquire the ability to invade distant organs. Liver X Receptors (LXRs) are nuclear receptors that have been demonstrated to regulate EMT in various cancers, including hepatic cancer. Our study reveals that the LXR pathway can control pro-invasive cell capacities through EMT in prostate cancer, employing ex vivo and in vivo approaches. We characterized the EMT status of the commonly used LNCaP, DU145, and PC3 prostate cancer cell lines through molecular and immunohistochemistry experiments. The impact of LXR activation on EMT function was also assessed by analyzing the migration and invasion of these cell lines in the absence or presence of an LXR agonist. Using in vivo experiments involving NSG-immunodeficient mice xenografted with PC3-GFP cells, we were able to study metastatic spread and the effect of LXRs on this process. LXR activation led to an increase in the accumulation of Vimentin and Amphiregulin in PC3. Furthermore, the migration of PC3 cells significantly increased in the presence of the LXR agonist, correlating with an upregulation of EMT. Interestingly, LXR activation significantly increased metastatic spread in an NSG mouse model. Overall, this work identifies a promoting effect of LXRs on EMT in the PC3 model of advanced prostate cancer.
Keywords: epithelial–mesenchymal transition; liver X receptors; metastasis; prostate cancer.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in study design, data collection and analysis, publishing management or manuscript writing and editing.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

