Advances in Personalized Oncology
- PMID: 39199633
- PMCID: PMC11352922
- DOI: 10.3390/cancers16162862
Advances in Personalized Oncology
Abstract
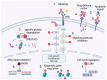
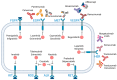
Advances in next-generation sequencing (NGS) have catalyzed a paradigm shift in cancer treatment, steering the focus from conventional, organ-specific protocols to precision medicine. Emerging targeted therapies offer a cutting-edge approach to cancer treatment, while companion diagnostics play an essential role in aligning therapeutic choices with specific molecular changes identified through NGS. Despite these advances, interpreting the clinical implications of a rapidly expanding catalog of genetic mutations remains a challenge. The selection of therapies in the presence of multiple mutations requires careful clinical judgment, supported by quality-centric genomic testing that emphasizes actionable mutations. Molecular tumor boards can play an increasing role in assimilating genomic data into clinical trials, thereby refining personalized treatment approaches and improving patient outcomes.
Keywords: driver mutations; oncogenic alterations; oncogenic drivers; personalized medicine; precision medicine; precision oncology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Druker B.J., Talpaz M., Resta D.J., Peng B., Buchdunger E., Ford J.M., Lydon N.B., Kantarjian H., Capdeville R., Ohno-Jones S., et al. Efficacy and Safety of a Specific Inhibitor of the BCR-ABL Tyrosine Kinase in Chronic Myeloid Leukemia. N. Engl. J. Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

