Current Knowledge and Perspectives of Immunotherapies for Neuroblastoma
- PMID: 39199637
- PMCID: PMC11353182
- DOI: 10.3390/cancers16162865
Current Knowledge and Perspectives of Immunotherapies for Neuroblastoma
Abstract
Neuroblastoma (NBL) cells highly express disialoganglioside GD2, which is restricted and weakly expressed in selected healthy cells, making it a desirable target of immunotherapy. Over the past two decades, application of dinutuximab, an anti-GD2 monoclonal antibody (mAb), has been one of the few new therapies to substantially improve outcomes to current levels. Given the persistent challenge of relapse and therapeutic resistance, there is an urgent need for new effective and tolerable treatment options for high-risk NBL. Recent breakthroughs in immune checkpoint inhibitor (ICI) therapeutics have not translated into high-risk NBL, like many other major pediatric solid tumors. Given the suppressed tumor microenvironment (TME), single ICIs like anti-CTLA4 and anti-PD1 have not demonstrated significant antitumor response rates. Meanwhile, emerging studies are reporting novel advancements in GD2-based therapies, targeted therapies, nanomedicines, and other immunotherapies such as adoptive transfer of natural killer (NK) cells and chimeric antigen receptors (CARs), and these hold interesting promise for the future of high-risk NBL patient care. Herein, we summarize the current state of the art in NBL therapeutic options and highlight the unique challenges posed by NBL that have limited the successful adoption of immune-modifying therapies. Through this review, we aim to direct the field's attention to opportunities that may benefit from a combination immunotherapy strategy.
Keywords: immunosuppression; immunotherapy; neuroblastoma; tumor microenvironment.
Conflict of interest statement
All authors declare no potential conflicts of interest.
Figures
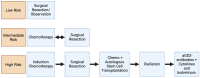
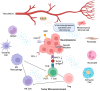
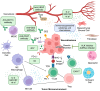
References
-
- Cohn S.L., Pearson A.D., London W.B., Monclair T., Ambros P.F., Brodeur G.M., Faldum A., Hero B., Iehara T., Machin D., et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J. Clin. Oncol. 2009;27:289–297. doi: 10.1200/JCO.2008.16.6785. - DOI - PMC - PubMed
-
- DuBois S.G., Kalika Y., Lukens J.N., Brodeur G.M., Seeger R.C., Atkinson J.B., Haase G.M., Black C.T., Perez C., Shimada H., et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J. Pediatr. Hematol. Oncol. 1999;21:181–189. doi: 10.1097/00043426-199905000-00005. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

