Real-World Outcomes of Atezolizumab with Bevacizumab Treatment in Hepatocellular Carcinoma Patients: Effectiveness, Esophagogastroduodenoscopy Utilization and Bleeding Complications
- PMID: 39199649
- PMCID: PMC11352899
- DOI: 10.3390/cancers16162878
Real-World Outcomes of Atezolizumab with Bevacizumab Treatment in Hepatocellular Carcinoma Patients: Effectiveness, Esophagogastroduodenoscopy Utilization and Bleeding Complications
Abstract
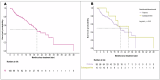
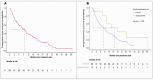
The IMbrave150 trial established atezolizumab with bevacizumab (A+B) as standard care for hepatocellular carcinoma (HCC), recommending an esophagogastroduodenoscopy (EGD) within 6 months of treatment initiation to prevent bleeding from esophagogastric varices. The necessity of mandatory EGD for all patients remains unclear. We retrospectively analyzed 112 HCC patients treated with A+B at five Canadian cancer centers from 1 July 2020 to 31 August 2022. A+B was the first-line therapy for 90% of patients, with median overall survival at 20.3 months and progression-free survival at 9.6 months. There was no survival difference between patients with bleeding and those without. Before A+B, 71% (n = 79) of patients underwent an EGD within 6 months, revealing varices in 41% (n = 32) and requiring intervention in 19% (n = 15). The overall bleeding rate was 15% (n = 17), with GI-specific bleeding occurring in 5% (n = 17). In the EGD group, GI-specific bleeding was 6% (n = 5) while in the non-EGD group, it was 3% (n = 1). Non-GI bleeding was observed in 10% (n = 11) of patients. Outcomes for HCC patients treated with A+B in Canada were comparable to IMbrave150. There was no increase in GI bleeding in patients without pre-treatment EGD, possibly supporting a selective EGD approach.
Keywords: EGD; GI bleeding; atezolizumab; bevacizumab; hepatocellular carcinoma; varices.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
LinkOut - more resources
Full Text Sources

